The viral safety testing market is valued at USD 899.8 million in 2025 and is projected to reach USD 3,736.4 million by 2035, growing at a CAGR of 15.3%. The initial acceleration phase demonstrates remarkable momentum, with market value increasing from USD 1,037.5 million in 2026 to USD 2,114.1 million by 2030. This foundational period marks an increase in biopharmaceutical development activities and the expansion of regulatory compliance requirements across both emerging and developed pharmaceutical markets.
The exponential growth phase is characterized by extraordinary market dynamics, propelling the market from USD 2,437.6 million in 2031 to reach USD 3,736.4 million by 2035. Dollar increments during the 2030-2035 period substantially exceed those of the earlier period, with annual value additions averaging USD 324.5 million compared to USD 242.9 million in the foundational phase. This progression represents a 315.2% total value increase over the forecast decade.
Market expansion drivers include accelerating biopharmaceutical manufacturing volumes, stringent viral safety regulatory requirements, and advanced testing methodology development. The 15.3% compound annual growth rate positions market participants to capitalize on USD 2,836.6 million in additional value creation opportunities. This trajectory signals exceptional prospects for testing service providers, technology innovators, and pharmaceutical quality assurance specialists across the global biopharmaceutical landscape.
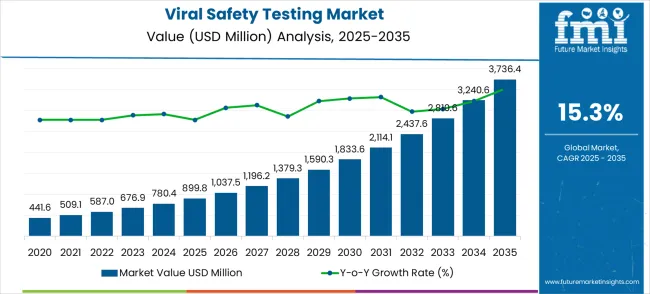
Market expansion unfolds through two distinct high-growth phases, characterized by accelerating technological advancements and development patterns. The 2025-2030 establishment period delivers USD 1,214.3 million in value additions, representing 135.0% growth from the baseline position. Market dynamics during this phase center on regulatory harmonization, technology standardization, and capacity expansion acceleration across pharmaceutical manufacturing and contract testing organizations worldwide.
The 2030-2035 acceleration period generates USD 1,622.3 million in incremental value, reflecting 76.7% growth from the 2030 position. This phase exhibits mature market characteristics with enhanced competition, specialized service differentiation strategies, and geographic expansion initiatives into emerging pharmaceutical markets. Value contributions shift from foundational capacity development to advanced testing methodology implementation and next-generation viral detection technology integration.
The competitive landscape evolves from emerging service development to established market leadership positioning. The initial period emphasizes the establishment of technology standards and regulatory compliance within traditional pharmaceutical testing applications. The subsequent period witnesses intensified competition for specialized testing segments and emerging biopharmaceutical market penetration. Market maturation factors include standardized testing protocols, automated analysis integration capabilities, and cross-application development across gene therapy, cell therapy, and advanced biologics manufacturing sectors.
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 899.8 million |
| Market Forecast (2035) ↑ | USD 3,736.4 million |
| Growth Rate ★ | 15.3% |
| Leading Product Type → | Adventitious virus tests |
| Primary Application → | Biopharmaceuticals |
Market expansion rests on five fundamental shifts driving biopharmaceutical quality assurance demand acceleration:
The growth faces challenges from high testing costs and complex regulatory requirements. Technical expertise limitations create capacity constraints for smaller testing organizations without specialized knowledge. Regulatory complexity increases operational expenses through extensive validation requirements and quality system maintenance.
Primary Classification: Test Type Distribution
Secondary Breakdown: Application Categories
Geographic Segmentation: Regional Market Distribution
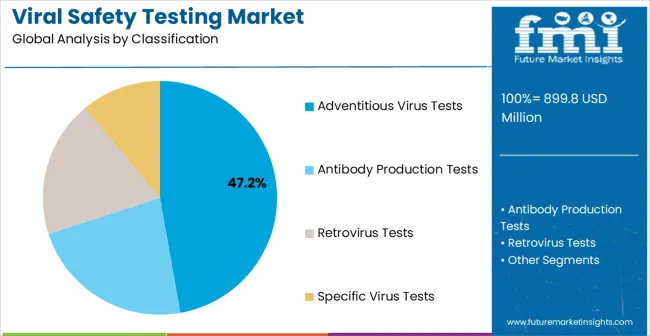
Market Position: Adventitious virus testing establishes clear market leadership through comprehensive viral contamination detection capabilities and regulatory compliance requirements. Adventitious testing systems eliminate unknown viral contamination risks in demanding biopharmaceutical manufacturing applications. Consistent detection capabilities enable comprehensive quality assurance where standard testing alternatives cannot achieve required sensitivity specifications.
Value Drivers: Advanced detection methodology improvements enhance viral identification accuracy and reduce false negative rates in critical applications. Digital analysis integration provides real-time contamination monitoring and automated result interpretation capabilities. Specialized testing protocols accommodate multiple product types within single analytical configurations for operational efficiency optimization.
Competitive Advantages: Adventitious testing offers comprehensive viral coverage compared to targeted alternatives requiring multiple specific assays for complete screening. Superior detection sensitivity increases product safety through early contamination identification in manufacturing processes. Premium pricing justification occurs through comprehensive risk mitigation benefits and regulatory compliance assurance.
Market Challenges: Complex testing protocols increase analytical costs compared to targeted viral testing approaches. Quality control requirements demand sophisticated laboratory equipment and technical expertise not available in all testing facilities. Extended testing timelines create supply chain challenges for time-sensitive pharmaceutical manufacturing operations.
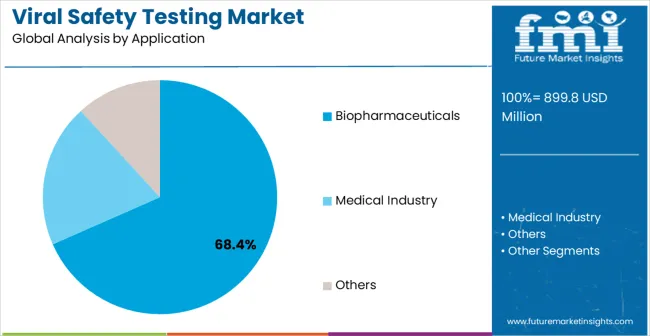
Strategic Market Importance: Biopharmaceutical manufacturing represents the primary demand driver for comprehensive viral safety testing across global pharmaceutical production facilities. Biologics production operations, monoclonal antibody manufacturing, and advanced therapeutic development require precise viral screening for regulatory approval and patient safety. Quality control standards mandate consistent testing performance for production optimization and product quality maintenance.
Market Dynamics Q&A:
Business Logic: Biopharmaceutical manufacturing facilities prioritize product safety and regulatory compliance, making comprehensive viral safety testing essential for meeting production standards. Cost justification occurs through contamination prevention and regulatory approval acceleration. Quality assurance requirements demand consistent testing protocols for optimal manufacturing performance and patient safety.
Forward-looking Implications: Advanced therapeutic development creates new requirements for specialized viral safety testing with enhanced detection capabilities. Personalized medicine manufacturing demands customized testing protocols for individualized therapeutic products. Global biopharmaceutical manufacturing growth sustains testing demand across expanding production regions with developing pharmaceutical infrastructure.
The viral safety testing market is primarily driven by increasing global pharmaceutical production requiring superior contamination detection and comprehensive quality assurance capabilities. Applications in biopharmaceutical manufacturing, gene therapy development, and advanced biologics production necessitate testing services that maintain rigorous detection standards under demanding regulatory conditions, making comprehensive viral safety testing an essential solution. The integration of advanced analytical technologies into pharmaceutical quality control operations further emphasizes the need for precise contamination detection and systematic risk assessment, ensuring consistent product safety. Stringent regulatory requirements across global pharmaceutical sectors encourage the use of comprehensive testing services that meet international safety standards and reduce contamination risks. Viral safety testing compliance with FDA, EMA, and other regulatory standards also makes it a preferred choice for critical pharmaceutical applications, where documented safety performance is essential for regulatory approval and patient protection, creating a stable demand pipeline globally.
Despite its advantages, the viral safety testing market faces several growth inhibitors. Testing service costs are significantly higher than conventional quality control methods, discouraging adoption in cost-sensitive pharmaceutical operations and smaller biotechnology companies. The testing and validation processes are complex, requiring specialized laboratory facilities, technical expertise, and sophisticated analytical systems, which are not universally available. Limited availability of qualified testing personnel and specialized equipment further constrains testing capacity expansion. The technical complexity involved in performing and interpreting viral safety tests necessitates continuous training programs and specialized quality systems, adding to operational overheads. Regulatory compliance requirements collectively slow market penetration, particularly in emerging pharmaceutical regions or resource-limited sectors, restraining overall market growth despite rising biopharmaceutical industry demand.
The viral safety testing market is evolving rapidly, driven by technological innovations and pharmaceutical industry advancement. Next-generation sequencing integration allows comprehensive viral detection capabilities, enhancing testing accuracy for specialized applications such as gene therapy products, cell therapy manufacturing, and advanced biological therapeutics. Automation in testing operations is another trend, reducing analysis costs, improving result consistency, and minimizing human error in complex analytical procedures. Digital transformation and data analytics initiatives are gaining traction, prompting the development of integrated testing platforms that enhance efficiency and provide comprehensive contamination monitoring. There is increasing demand for specialized testing services tailored to specific pharmaceutical applications, offering operational flexibility while maintaining regulatory compliance standards. These trends collectively indicate a market that is technologically advancing while responding to regulatory, safety, and application-specific needs, ensuring sustained growth and innovation.
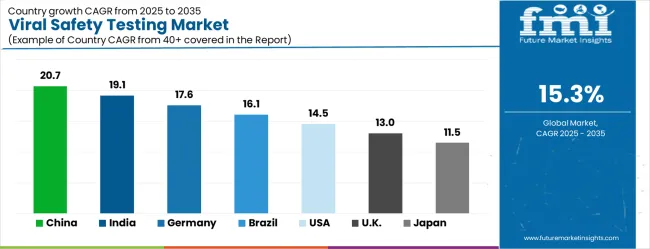
Global market dynamics reveal distinct performance tiers reflecting regional pharmaceutical manufacturing capabilities and biotechnology industry development levels. Growth Leaders including China (20.7% CAGR) and India (19.1% CAGR) demonstrate expanding biopharmaceutical production ecosystems with advanced testing technology adoption, while Germany (17.6% CAGR) represents European pharmaceutical excellence in quality assurance applications. Strong Performers such as Brazil (16.1% CAGR) and the United States (14.5% CAGR) show robust demand growth aligned with pharmaceutical industry modernization and testing capacity requirements. Mature Markets including the United Kingdom (13.0% CAGR) and Japan (11.5% CAGR) display solid growth rates reflecting established market penetration and advanced testing technology focus.
Regional synthesis indicates Asia Pacific dominance through pharmaceutical manufacturing concentration and export production requirements. European markets emphasize quality standards and regulatory compliance in pharmaceutical applications. North American demand reflects biotechnology innovation advancement and specialized testing development initiatives.
| Country | CAGR (%) |
|---|---|
| China | 20.7 |
| India | 19.1 |
| Germany | 17.6 |
| Brazil | 16.1 |
| U.S. | 14.5 |
| U.K. | 13.0 |
| Japan | 11.5 |
China establishes market leadership through biopharmaceutical manufacturing sector dominance across biologics production facilities, pharmaceutical manufacturing operations, and contract research organizations. A 20.7% CAGR value reflects extensive production capacity and export-oriented pharmaceutical manufacturing requirements driving exceptional demand for advanced viral safety testing services. Growth metrics demonstrate comprehensive pharmaceutical industry modernization supported by government healthcare development initiatives and stringent quality improvement mandates across biopharmaceutical sectors.
Market dynamics center on domestic pharmaceutical production expansion creating new high-quality testing requirements for enhanced product safety and substantial regulatory compliance targets. Government pharmaceutical industry development programs drive widespread adoption of advanced testing services for operational quality improvement and international market competitiveness. Export market demands necessitate strict international quality standard adherence through extensively documented testing specifications and certification processes.
Manufacturing concentration in major pharmaceutical producing regions creates significant demand density for comprehensive viral safety testing services and specialized applications. Industrial development policies specifically include advanced testing requirements for comprehensive pharmaceutical quality upgrading initiatives. Foreign investment partnerships bring international pharmaceutical standards requiring advanced viral safety testing applications and technical expertise development.
India demonstrates exceptional growth potential through rapidly expanding pharmaceutical manufacturing capabilities and comprehensive government promotion of domestic biopharmaceutical development initiatives under various healthcare advancement programs. A 19.1% CAGR reflects increasing pharmaceutical sophistication and widespread quality standard adoption across biopharmaceutical manufacturing sectors. Pharmaceutical industry growth under government initiatives creates remarkable demand for advanced viral safety testing services across both integrated pharmaceutical complexes and specialized biotechnology facilities.
Contract manufacturing sector expansion drives significant secondary demand through stringent quality requirements for international pharmaceutical production and export applications. Government pharmaceutical certification mandates actively promote advanced testing service adoption for enhanced safety compliance and international standard adherence. Major healthcare development projects substantially increase pharmaceutical production volumes requiring comprehensive viral safety testing applications and specialized quality assurance systems.
Market activity centers on extensive technology transfer agreements bringing international pharmaceutical testing standards and best practices to domestic manufacturing facilities. Foreign direct investment in pharmaceutical operations introduces advanced viral safety testing requirements for improved operational quality and competitive advantage. Skill development initiatives include comprehensive training programs for advanced testing methodologies and quality control procedures.
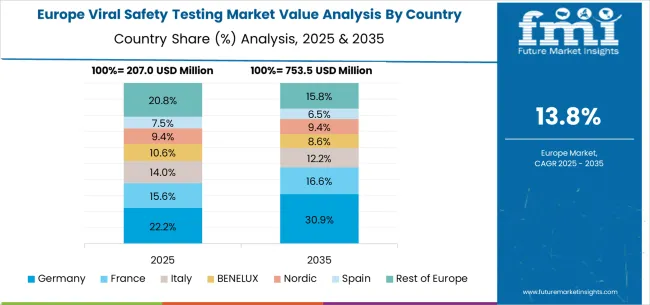
Germany maintains market leadership through exceptional pharmaceutical manufacturing excellence and established biotechnology research traditions across diverse healthcare sectors. A 17.6% CAGR demonstrates robust market demand supported by an advanced pharmaceutical industrial base with sophisticated biopharmaceutical production capabilities and stringent regulatory compliance requirements. Pharmaceutical sector strength creates consistent demand for comprehensive viral safety testing services across precision drug manufacturing and advanced biologics development applications requiring superior analytical capabilities.
Biotechnology research sector requires extensively certified testing specifications for strict export compliance and international pharmaceutical quality standards adherence. Contract research organizations utilize premium testing services for advanced drug development and comprehensive safety evaluation applications requiring precise analytical characteristics. Quality standard development significantly influences global pharmaceutical practices requiring advanced testing adoption and technological innovation across European markets.
Advanced pharmaceutical research initiatives continuously develop innovative applications for viral safety testing across emerging therapeutic sectors. Export market leadership necessitates comprehensive analytical documentation supporting international pharmaceutical sales and regulatory compliance requirements across global healthcare markets.
Brazil represents a significant emerging pharmaceutical market with substantial growth potential, achieving a 16.1% CAGR through comprehensive healthcare development initiatives and pharmaceutical manufacturing expansion across domestic and regional markets. The market reflects increasing pharmaceutical sophistication and widespread quality standard adoption driven by government policies promoting domestic biopharmaceutical production capabilities. Pharmaceutical industry modernization creates consistent demand for advanced viral safety testing services across integrated pharmaceutical facilities and specialized biotechnology installations.
Generic pharmaceutical manufacturing growth drives primary market demand through domestic production expansion and regional export requirements for Latin American healthcare markets. Contract manufacturing sector expansion creates substantial opportunities for precision testing applications and advanced quality assurance solutions. Healthcare sector development significantly increases pharmaceutical production volumes requiring comprehensive analytical capabilities and advanced testing systems.
Market development faces challenges including regulatory harmonization affecting pharmaceutical investment decisions and currency fluctuation impacting imported testing equipment costs. Healthcare infrastructure development projects support pharmaceutical sector growth requiring precision analytical capabilities and advanced testing applications across expanding production networks.
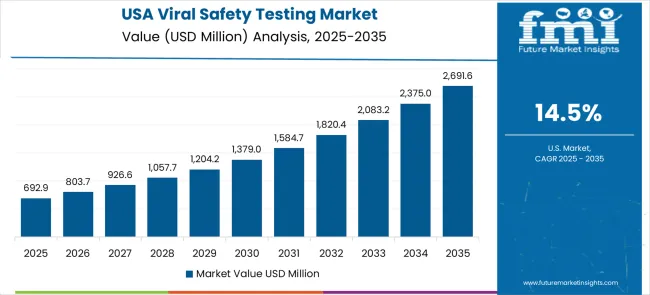
The United States market demonstrates exceptional and sustained growth with a 14.5% CAGR through comprehensive biotechnology sector leadership and strategic pharmaceutical innovation initiatives driving advanced therapeutic development. The market reflects an established pharmaceutical industrial base with significant research advancement trends and cutting-edge biopharmaceutical capabilities. Biotechnology sector leadership creates substantial demand for advanced viral safety testing services and comprehensive analytical systems meeting stringent FDA regulatory requirements.
Gene therapy industry leadership requires precision testing applications for critical therapeutic development and specialized manufacturing procedures requiring advanced analytical protocols. Pharmaceutical research sector maintains consistent demand for quality-certified testing procedures and comprehensive analytical specifications supporting innovative drug development. Biopharmaceutical automation adoption creates sophisticated requirements for testing service integration with advanced manufacturing execution systems.
Pharmaceutical competitiveness initiatives encourage substantial investment in advanced testing services and comprehensive analytical technologies supporting innovation advancement. Regulatory compliance requires extensively documented testing procedures supporting FDA approval processes and international market access requirements across global pharmaceutical distribution networks.
The United Kingdom demonstrates strong market stability through comprehensive pharmaceutical innovation and strategic biotechnology advancement initiatives across key healthcare sectors. A 13.0% CAGR market value reflects an established pharmaceutical manufacturing base with strong emphasis on regulatory excellence and precision drug development applications. Biotechnology sector strength creates substantial demand for precision viral safety testing applications in critical research operations and advanced therapeutic manufacturing procedures.
Pharmaceutical development sector requires precision testing services for drug manufacturing and regulatory approval operations meeting stringent MHRA quality and safety standards. Contract research organizations maintain strict quality standard requirements for international pharmaceutical markets and global competitiveness supporting advanced drug development. Pharmaceutical research initiatives continuously develop innovative applications for precision testing services and comprehensive analytical systems.
Post-Brexit pharmaceutical considerations create significant opportunities for domestic testing capacity growth requiring precision analytical capabilities and advanced testing solutions supporting pharmaceutical independence. Government pharmaceutical strategy initiatives actively support advanced testing technology adoption and comprehensive innovation development across biotechnology sectors.
Japan maintains technological leadership through exceptional precision pharmaceutical manufacturing excellence and comprehensive quality standard development across diverse healthcare sectors. An 11.5% CAGR market value reflects a mature pharmaceutical market with established precision testing service adoption across industries and advanced analytical capabilities supporting innovation. Pharmaceutical sector leadership creates consistent demand for advanced testing technologies and comprehensive viral safety testing services meeting strict regulatory quality requirements.
Biotechnology manufacturing sector requires precision testing services for therapeutic development and comprehensive analytical applications ensuring reliable pharmaceutical performance across global markets. Medical device industry utilizes specialized testing applications for precision healthcare manufacturing and comprehensive quality procedures supporting advanced therapeutic delivery systems. Quality management system development significantly influences global pharmaceutical practices and analytical technology adoption standards.
Pharmaceutical technology exports include precision testing service requirements for international production facilities and comprehensive technology transfer programs supporting global healthcare advancement. Research and development initiatives continuously advance testing methodologies and comprehensive analytical technologies supporting pharmaceutical innovation across emerging therapeutic applications.
The viral safety testing market in Europe is projected to experience robust growth, supported by strong pharmaceutical industry demand for advanced quality assurance services. The market value in Europe is estimated at USD 287.4 million in 2025, with Germany leading the region with a 38.7% share, driven by its robust pharmaceutical manufacturing industry and advanced biotechnology research capabilities. The United Kingdom follows with a 21.3% market share, benefiting from pharmaceutical innovation initiatives and stringent regulatory standards in drug development and biologics manufacturing. France holds 16.8% of the market, supported by expansions in biopharmaceutical research infrastructure and precision medicine investments requiring comprehensive viral safety testing. Italy and Spain collectively represent 14.9% of European demand, spurred by pharmaceutical manufacturing development and growing adoption of advanced testing methodologies. The Rest of Europe, including Nordic countries, BENELUX region, and Eastern European nations, accounts for 8.3% of the market, backed by specialized pharmaceutical applications and expanding biotechnology research operations. This diverse regional distribution highlights Europe's significance as a key market for viral safety testing services, driven by evolving pharmaceutical regulations, biotechnology advancement requirements, and stringent quality standards across multiple sectors including pharmaceuticals, biotechnology, medical devices, and contract research organizations.
The Viral Safety Testing market is defined by competition among specialized contract research organizations, pharmaceutical testing service providers, and comprehensive analytical solution companies. Companies are investing in advanced testing methodology development, regulatory compliance optimization, analytical accuracy improvements, and comprehensive service capabilities to deliver reliable, precise, and regulatory-compliant viral contamination detection solutions. Strategic partnerships, technological innovation, and market expansion are central to strengthening service portfolios and market presence.
Charles River offers comprehensive viral safety testing solutions with established analytical expertise and industry-leading regulatory compliance capabilities for large-scale pharmaceutical operations. Eurofins BioPharma provides specialized testing services with focus on operational efficiency and advanced analytical method integration. Clean Cells delivers advanced testing technology solutions with emphasis on contamination detection reliability and analytical optimization. Texcell specializes in professional viral safety services with advanced testing protocol integration and comprehensive analytical capabilities.
Merck Millipore offers precision testing equipment with comprehensive pharmaceutical industry support capabilities. Syngene delivers established viral safety testing solutions with advanced analytical technologies and cost-effective implementations. Nelson Labs provides specialized testing services with focus on regulatory compliance optimization. WuXi Biologics, Laboratory Corporation of America Holdings, Creative Biogene, and Asahi Kasei Corporation offer specialized analytical expertise, testing reliability, and comprehensive service development across global and regional market segments.
The viral safety testing market is central to pharmaceutical safety excellence, biopharmaceutical quality assurance, regulatory compliance advancement, and patient protection initiatives. With increasing demands for testing accuracy, regulatory harmonization, and operational efficiency, the sector faces pressure to balance testing costs, analytical complexity, and service accessibility measures. Coordinated action from governments, regulatory bodies, testing service providers, pharmaceutical companies, and investors is essential to transition toward technologically advanced, globally standardized, and comprehensively accessible viral safety testing systems.
How Governments Could Spur Local Testing Infrastructure and Adoption?
How Regulatory Bodies Could Support Market Development?
How Testing Service Providers Could Strengthen the Ecosystem?
How Pharmaceutical Companies Could Navigate the Quality Shift?
How Investors and Financial Enablers Could Unlock Value?
| Item | Value |
|---|---|
| Quantitative Units | USD 899.8 million |
| Test Type | Adventitious Virus Tests, Antibody Production Tests, Retrovirus Tests, Specific Virus Tests |
| Application | Biopharmaceuticals, Medical Industry, Others |
| Regions Covered | North America, Latin America, Europe, East Asia, South Asia & Pacific, Middle East & Africa |
| Countries Covered | United States, Canada, Mexico, Germany, United Kingdom, France, Italy, Spain, Nordic, BENELUX, China, Japan, South Korea, India, ASEAN, Australia, New Zealand, Brazil, Chile, Kingdom of Saudi Arabia, GCC Countries, Turkey, South Africa |
| Key Companies Profiled | Charles River, Eurofins BioPharma, Clean Cells, Texcell, Merck Millipore, Syngene, Nelson Labs, Sartorius Stedim BioOutsource Limited, WuXi Biologics, ViSpot Co., Ltd., Syngene International Ltd., Laboratory Corporation of America Holdings, Creative Biogene, Asahi Kasei Corporation |
| Additional Attributes | Dollar sales by test type categories, regional demand trends across Asia Pacific, Europe, and North America, competitive landscape with service provider descriptions, adoption patterns across biopharmaceutical and medical industries, integration with pharmaceutical manufacturing, innovations in testing technology and analytical control, and development of specialized applications with enhanced detection capabilities |
The global viral safety testing market is estimated to be valued at USD 899.8 million in 2025.
The market size for the viral safety testing market is projected to reach USD 3,736.4 million by 2035.
The viral safety testing market is expected to grow at a 15.3% CAGR between 2025 and 2035.
The key product types in viral safety testing market are adventitious virus tests, antibody production tests, retrovirus tests and specific virus tests.
In terms of application, biopharmaceuticals segment to command 68.4% share in the viral safety testing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Viral Molecular Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Viral Vector Development Market – Growth & Demand 2025 to 2035
Viral RNA Extraction Kit Market - Trends & Forecast 2025 to 2035
Viral Pneumonia Treatment Market
Antiviral Drugs Market Size and Share Forecast Outlook 2025 to 2035
Antiviral Immunoglobulin Market Growth – Trends & Forecast 2025 to 2035
Antiviral Drug Packaging Market Insights - Growth & Trends 2025 to 2035
Antiviral Polymers for Packaging Market
HIV Antivirals Market Size and Share Forecast Outlook 2025 to 2035
Swab and Viral Transport Medium Market Insights - Demand & Forecast 2025 to 2035
Key Players & Market Share in Swab and Viral Transport Medium Industry
Influenza Antiviral Market
China Swab and Viral Transport Medium Market Trends – Size, Demand & Industry Outlook 2025-2035
Sterile and Antiviral Packaging Market Forecast and Outlook 2025 to 2035
France Swab and Viral Transport Medium Market Report - Growth, Trends & Forecast 2025 to 2035
Arthropod-borne Viral Infections Testing Market Size and Share Forecast Outlook 2025 to 2035
Germany Swab and Viral Transport Medium Market Insights - Demand, Size & Industry Trends 2025 to 2035
Direct-acting Antiviral Medicines Market
United States Swab and Viral Transport Medium Market Outlook - Size, Share & Industry Trends 2025 to 2035
United Kingdom Swab and Viral Transport Medium Market Analysis - Trends, Growth & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA