The Aseptic Formulation Processing Market is estimated to be valued at USD 10.5 billion in 2025 and is projected to reach USD 20.3 billion by 2035, registering a compound annual growth rate (CAGR) of 6.8% over the forecast period.
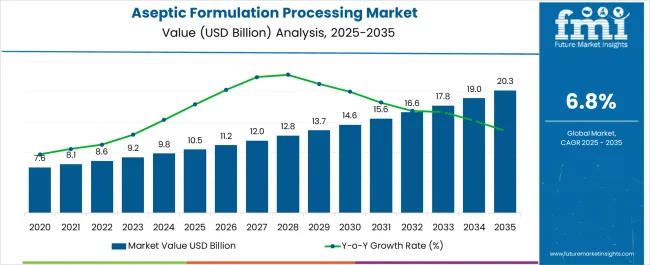
| Metric | Value |
|---|---|
| Aseptic Formulation Processing Market Estimated Value in (2025 E) | USD 10.5 billion |
| Aseptic Formulation Processing Market Forecast Value in (2035 F) | USD 20.3 billion |
| Forecast CAGR (2025 to 2035) | 6.8% |
The Aseptic Formulation Processing market is witnessing substantial growth due to increasing demand for sterile pharmaceutical products and vaccines, driven by heightened awareness of safety and quality standards. The market is being shaped by technological advancements in automated systems, which enable consistent sterile conditions, higher throughput, and reduced human intervention, minimizing contamination risks. Rising investments in pharmaceutical infrastructure, the growing prevalence of chronic diseases, and the expansion of biologics and vaccines are contributing to market growth.
The integration of process monitoring, real-time analytics, and advanced control systems is supporting enhanced productivity and regulatory compliance. Increasing regulatory scrutiny on contamination control and stringent quality standards is pushing manufacturers toward adopting automated aseptic processing technologies.
Additionally, the need for faster product development cycles, particularly in biologics and injectable therapeutics, has created opportunities for scalable, flexible processing platforms The market is expected to continue its upward trajectory as pharmaceutical companies seek efficient, compliant, and high-quality aseptic production solutions to meet growing global demand.
The aseptic formulation processing market is segmented by technology, product type, end user, and geographic regions. By technology, aseptic formulation processing market is divided into Automated, Semi-Automated, and Manual. In terms of product type, aseptic formulation processing market is classified into Laminar Air Flow Systems, Autoclave Devices, Dry Heat Ovens, UV- Radiation Systems, Aseptic Filling Devices, Open, Barrier, Isolators, and RABS (Restricted Access Barrier Systems). Based on end user, aseptic formulation processing market is segmented into Pharmaceutical Companies, Biotech Companies, Research Laboratories, and Others. Regionally, the aseptic formulation processing industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
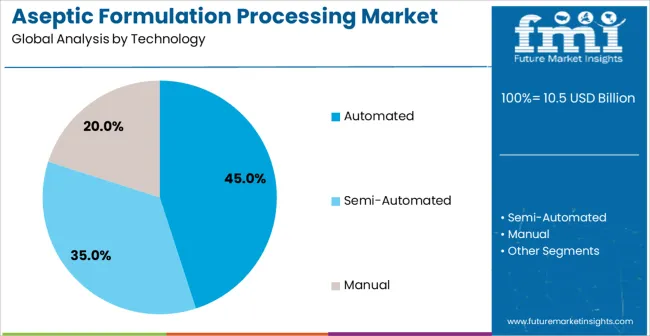
The Automated technology segment is projected to hold 45.0% of the Aseptic Formulation Processing market revenue share in 2025, making it the leading technology. This position is being attributed to the superior capability of automated systems to maintain consistent sterile conditions and reduce manual errors, which is critical for regulatory compliance and product safety.
Automation allows for real-time monitoring, precise control of environmental parameters, and efficient handling of sensitive formulations, which has driven adoption in high-volume pharmaceutical production facilities. The ability to integrate with advanced software systems for process optimization and documentation further enhances operational efficiency.
Pharmaceutical companies are increasingly favoring automated systems to meet stringent quality standards, reduce contamination risks, and improve productivity, making this segment a dominant contributor to market growth Future expansion is expected as manufacturers continue to upgrade existing facilities with advanced automation solutions to achieve higher throughput and regulatory adherence.
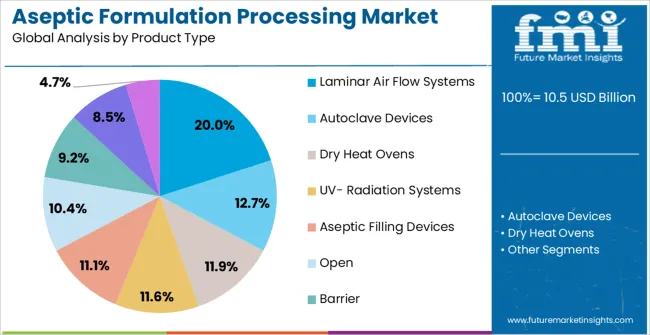
Laminar Air Flow Systems are projected to account for 20.0% of the overall Aseptic Formulation Processing market revenue share in 2025, establishing them as the leading product type. This prominence is being driven by their ability to provide a controlled sterile environment, which is critical for aseptic filling and formulation processes.
These systems ensure unidirectional airflow, reducing contamination risks and supporting compliance with regulatory standards in pharmaceutical manufacturing. The adoption of laminar airflow systems has been accelerated by the increasing production of injectable drugs, vaccines, and biologics that require stringent sterility.
The segment benefits from technological advancements that enhance airflow uniformity, energy efficiency, and integration with automation for real-time monitoring As pharmaceutical production scales up globally, the demand for reliable and efficient laminar air flow systems is expected to remain strong, supporting the segment’s leading position in the market.
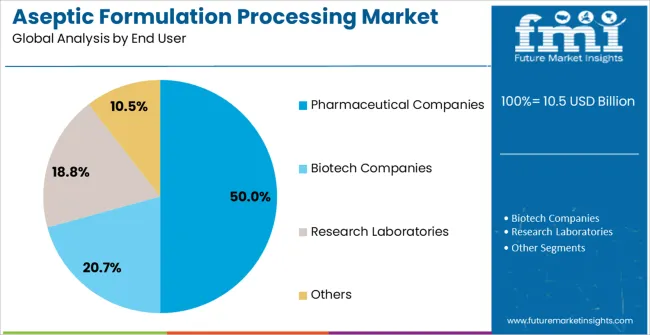
The Pharmaceutical Companies end-use industry segment is expected to hold 50.0% of the Aseptic Formulation Processing market revenue share in 2025, making it the largest end-user segment. This dominance is being driven by the growing need for sterile drug production, particularly in injectable therapies, biologics, and vaccines.
Pharmaceutical companies are increasingly adopting advanced aseptic processing technologies to ensure regulatory compliance, enhance product safety, and improve production efficiency. Investments in expanding manufacturing capacities, modernization of facilities, and integration of automation are enabling higher throughput and consistent product quality.
The segment’s growth is also supported by the rising prevalence of chronic diseases, growing demand for biologics, and global vaccination programs, which require large-scale sterile production As pharmaceutical companies prioritize cost-effective, scalable, and compliant aseptic solutions, the adoption of automated technologies and laminar air flow systems is expected to drive sustained growth in this segment.
Aseptic formulation processing is the manipulation of sterile active pharmaceutical ingredient (APIs) or pharmaceutical product in a carefully controlled environment using aseptic technique in order to produce a sterile end product.
Aseptic formulation processing is a risk management program that ensures the pharmaceutical product's safety and integrity by confirming the transfer of formulation ingredients with high potency without any chance of contamination in the pre-sterilized vials or containers.
Maintaining a controlled environment is vital in aseptic formulation processing as the quality of the final product and sterility of the manufacturing procedure is maintained due to the sterile environment. The environmental conditions required for aseptic processing is maintained by sterile air supply through laminar air flow machines in the aseptic formulation processing facilities.
During the process of packaging of the final product, technical measures should be taken such as handling sterile materials in a controlled environment in order to control microbial and particulate contamination to acceptable levels.
This process is done manually or with the help of automated aseptic filling devices. However, for improving the aseptic formulation processing procedures in the pharmaceutical industry, the Parenteral Drug Association (PDA) published its aseptic validation technical report in 1981. After that several regulatory authorities published guidelines for aseptic processing of pharmaceutical products.

| Country | CAGR |
|---|---|
| China | 9.2% |
| India | 8.5% |
| Germany | 7.8% |
| Brazil | 7.1% |
| USA | 6.5% |
| UK | 5.8% |
| Japan | 5.1% |
The Aseptic Formulation Processing Market is expected to register a CAGR of 6.8% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 9.2%, followed by India at 8.5%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Japan posts the lowest CAGR at 5.1%, yet still underscores a broadly positive trajectory for the global Aseptic Formulation Processing Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 7.8%. The USA Aseptic Formulation Processing Market is estimated to be valued at USD 3.6 billion in 2025 and is anticipated to reach a valuation of USD 3.6 billion by 2035. Sales are projected to rise at a CAGR of 0.0% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 484.6 million and USD 307.0 million respectively in 2025.

| Item | Value |
|---|---|
| Quantitative Units | USD 10.5 Billion |
| Technology | Automated, Semi-Automated, and Manual |
| Product Type | Laminar Air Flow Systems, Autoclave Devices, Dry Heat Ovens, UV- Radiation Systems, Aseptic Filling Devices, Open, Barrier, Isolators, and RABS (Restricted Access Barrier Systems) |
| End User | Pharmaceutical Companies, Biotech Companies, Research Laboratories, and Others |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
| Key Companies Profiled | Robert Bosch GmbH, Becton Dickinson and Company, Tetra Laval International S.A., E.I. du Pont de Nemours and Company, Sealed Air Corporation, GEA Group Aktiengesellschaft, Alfa Laval AB, and Krones AG |
The global aseptic formulation processing market is estimated to be valued at USD 10.5 billion in 2025.
The market size for the aseptic formulation processing market is projected to reach USD 20.3 billion by 2035.
The aseptic formulation processing market is expected to grow at a 6.8% CAGR between 2025 and 2035.
The key product types in aseptic formulation processing market are automated, semi-automated and manual.
In terms of product type, laminar air flow systems segment to command 20.0% share in the aseptic formulation processing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Aseptic IBC Market Size and Share Forecast Outlook 2025 to 2035
Aseptic Packaging Paperboard Market Size and Share Forecast Outlook 2025 to 2035
Aseptic Liquid Packaging Boards Market Size and Share Forecast Outlook 2025 to 2035
Aseptic Containment Systems Market Size and Share Forecast Outlook 2025 to 2035
Aseptic Packaging Market Size and Share Forecast Outlook 2025 to 2035
Aseptic Packaging Equipment Market Trends - Growth & Forecast 2025 to 2035
Aseptic Fillers Market Growth - Trends & Forecast 2025 to 2035
Market Share Insights of Leading Aseptic Carton Providers
Aseptic Paper for Packaging Market from 2023 to 2033
Aseptic Processing Market Growth - Trends & Forecast 2025 to 2035
Robotic Aseptic Syringe Filler Capper Market Size and Share Forecast Outlook 2025 to 2035
Gable Top Aseptic Cartons Market Size and Share Forecast Outlook 2025 to 2035
North America Bulk Aseptic Packaging Market Size and Share Forecast Outlook 2025 to 2035
Demand for Gable Top Aseptic Cartons in UK Size and Share Forecast Outlook 2025 to 2035
Demand for Gable Top Aseptic Cartons in USA Size and Share Forecast Outlook 2025 to 2035
Demand for Gable Top Aseptic Cartons in Middle East & Africa Size and Share Forecast Outlook 2025 to 2035
Formulation Development Outsourcing Market Size and Share Forecast Outlook 2025 to 2035
Preformulation intermediates Market Size and Share Forecast Outlook 2025 to 2035
Drug Formulation Market Analysis - Size, Growth, & Forecast Outlook 2025 to 2035
Bakuchiol Formulations Market Analysis - Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA