The castration-resistant prostate cancer (CRPC) industry is valued at USD 13.5 billion in 2025. As per FMI’s analysis, the castration-resistant prostate cancer (CRPC) industry will grow at a CAGR of 9.5% and reach USD 33.3 billion by 2035. This high-growth path is largely fueled by the increasing worldwide incidence of prostate cancer cases progressing to castration-resistant phases.
In 2024, the treatment paradigm of castration-resistant prostate cancer (CRPC) witnessed a dramatic shift, driven by accelerated research activities and swift integration of personalized medicine in clinical practice.
Pipeline development became pharmaceutical firms' top priority, with a focus on next-generation androgen receptor inhibitors and emerging radiopharmaceuticals. This realignment came in conjunction with a strategic move toward the incorporation of companion diagnostics, allowing for more accurate patient stratification and enhanced therapeutic response.
Looking forward to 2025, the CRPC treatment industry is set to witness rapid growth. The intersection of precision oncology, real-world evidence, and AI-powered decision-making tools will optimize treatment protocols and improve patient management. In addition, growing clinical adoption of combination therapies-especially those combining targeted agents with immunotherapies-will redefine standard care practices.
| Metric | Value |
|---|---|
| Industry Value (2025E) | USD 13.5 billion |
| Industry Value (2035F) | USD 33.3 billion |
| CAGR (2025 to 2035) | 9.5% |
The castration-resistant prostate cancer (CRPC) treatment industry is on a solid growth path, fueled by the increasing incidence of prostate cancer and innovation in targeted therapies. The growing use of precision medicine and combination therapies is transforming care delivery to the advantage of pharmaceutical innovators and diagnostics companies. Conventional monotherapy strategies could lose share as more effective, customized solutions become increasingly popular.
Drive Innovation in Targeted Therapies
Invest in the research and development of new-generation androgen receptor inhibitors, radiopharmaceuticals, and combination therapy to manage drug resistance and enhance clinical outcomes in CRPC patients.
Aspire towards Precision Medicine and Diagnostic Integration
Prioritize product pipeline alignment with the increasing need for individualized treatment by integrating genomic testing, biomarkers, and advanced imaging in therapeutic approaches.
Enhance Strategic Partnerships and Clinical Trials Network
Develop expanded partnerships among biotech companies, academia, and clinical research organizations to drive R&D more rapidly, simplify regulatory routes, and improve industry access through collaborative commercialization.
| Risk | Probability - Impact |
|---|---|
| Delayed regulatory approvals for new therapies: Extended approval times can delay the commercialization of innovative treatments, slowing industry momentum. | Medium - High |
| High cost of treatment constraining patient accessibility in developing economies: Costly treatments can limit uptake, particularly in low-resource economies without reimbursement. | High - Medium |
| Limited long-term efficacy information for recent combination regimens: Lack of post-marketing data can raise concerns regarding longer-term benefits, impacting clinician confidence and uptake. | Medium - Medium |
| Priority | Immediate Action |
|---|---|
| Diversify and fortify the therapeutic pipeline. | Perform feasibility studies and early-stage trials for next-generation androgen receptor antagonists and radiopharmaceuticals against advanced CRPC pathways. |
| Advanced diagnostic-therapy convergence | Build strategic partnerships with diagnostic companies to co-develop companion diagnostics such as genomic profiling and liquid biopsy tests for the early detection of and monitoring of CRPC. |
| Expand global access and commercialization reach | Develop region-by-region pricing structures and reimbursement models; launch stakeholder engagement programs in under-penetrated sectors to drive therapy adoption. |
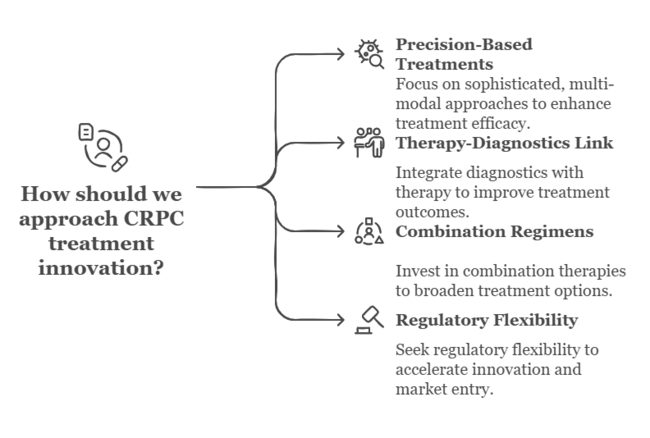
To stay ahead, companies must swiftly shift to precision-based, multi-modal treatment approaches that capture the increasing sophistication of castration-resistant prostate cancer (CRPC). This insight emphasizes the imperative to bring R&D in sync with genomic and biomarker-led innovation, even as it shores up access approaches through value-based pricing and focused global expansion.
Companies that link therapy with diagnostics, invest in combination regimens, and work collaboratively across the oncology ecosystem will be most well placed to realize long-term value. Board-level attention should now turn towards speeding up product pipelines, broadening clinical trial landscapes, and winning regulatory flexibility to lead the next wave of CRPC treatment innovation.
Hormonal therapy is anticipated to be the most profitable therapy type in the sector for castration-resistant prostate cancer (CRPC) treatment between 2025 and 2035. This segment is likely to grow at a CAGR of 10.2%, higher than the global average, because of the extensive clinical usage of sophisticated androgen receptor signaling inhibitors like enzalutamide, apalutamide, and abiraterone. These drugs continue to show substantial efficacy in slowing disease progression and enhancing survival rates, even in metastatic disease.
Their oral route of administration, safety profile relative to chemotherapy, and increasing use in combination with other modalities such as radiopharmaceuticals add to their popularity among patients and clinicians alike.
Non-steroidal antiandrogens are projected to dominate CRPC drug class sales between 2025 and 2035, with a CAGR of 10.5%, above the global average. This dominance is fueled by sustained clinical choice for drugs such as enzalutamide, apalutamide, and darolutamide, which potently inhibit androgen receptor signaling-a critical driver of CRPC growth.
These agents are increasingly being used as first-line and second-line therapies because they can prolong survival with tolerable side effects. Their use in combination regimens with corticosteroids or chemotherapy is also growing, further increasing treatment customization and efficacy.
The large number of active clinical trials and next-generation pipeline molecules in this class further solidifies its leadership and long-term growth potential in the emerging CRPC treatment paradigm.
The oral route is anticipated to continue being the most profitable drug delivery route for castration-resistant prostate cancer (CRPC) therapies between 2025 and 2035, growing at a CAGR of 10.1%. This is due to the extensive use of orally active non-steroidal antiandrogens like enzalutamide and abiraterone, which are currently the cornerstone treatments in CRPC management.
Oral therapies provide improved patient compliance, convenience of administration in the outpatient setting, and lower healthcare costs related to hospital-based infusions. With the CRPC therapeutic environment moving increasingly toward chronic disease management and home care, the need for oral agents will increase substantially.
Hospital pharmacies are expected to be the most profitable distribution channel for CRPC treatments from 2025 to 2035, expanding at a CAGR of 9.8%. The channel leads because CRPC management is complicated and usually needs multidisciplinary management, combination regimens, and access to expensive therapies under clinical supervision.
Hospital pharmacies are key to ensuring the timely delivery of advanced hormonal agents, chemotherapy, radiopharmaceuticals, and immunotherapies, particularly in metastatic or advanced cases. Regulatory compliance, reimbursement procedures, and clinical trial enrollment are also better managed within hospital-based systems.
The United States will be a leader in the CRPC treatment landscape, supported by advanced oncology infrastructure, high treatment availability, and early uptake of new therapeutics. Precision medicine is a key thrust, with extensive use of genomic profiling and companion diagnostics for targeting therapy selection.
Pharmaceutical firms are in a race to launch next-generation antiandrogens and radioligand therapies, with the FDA's accelerated approval pathway remaining a key driver for fast-track drug development.
Reimbursement systems are strong, particularly for expensive treatments, and the USA provides an optimum launch platform for international drug launches. Clinical trial take-up is also above global standards, providing real-world learning that further refines treatment pathways. FMI forecasts that the CAGR of the United States is 10.1% from 2025 to 2035.
India's CRPC treatment scenario is changing, spurred by a rise in the incidence of prostate cancer, growing health penetration, and government initiatives to enhance accessibility of cancer care.
Although late-stage diagnosis continues to pose a challenge, urbanization and enhanced diagnostic outreach are promoting earlier treatment. The base of generic drug manufacturing is allowing cost-efficient therapy, especially hormone therapy and chemotherapeutics.
Medical tourism also favors the industry, with India charging much lower costs of treatment in comparison to the West. The emergence of online pharmacies and tele-oncology platforms is also increasing CRPC treatments. FMI is of the opinion that India's CAGR will be 9.2% from 2025 to 2035.
China is becoming a prominent growth driver for CRPC therapies due to aggressive healthcare reform, demographic changes, and expanding cancer awareness. The expansion of oncology centers is supported by the government's "Healthy China 2030" policy, which accelerates diagnosis and treatment access. National funding-backed local biotech companies are launching new molecular entities for CRPC, minimizing imports.
The growing adoption of PSA screening and genomic testing has resulted in increasing early detection rates, allowing for timely treatment. Reforms in regulation in China, particularly expedited approvals through the National Medical Products Administration (NMPA), are driving innovation. FMI forecasts that the CAGR of China is 10.3% from 2025 to 2035.
The UK CRPC treatment industry is picking up pace with NHS-led universal access and aggressive oncology policies. Early screening and accelerated treatment are priorities of the government's Cancer Strategy, resulting in increasing adoption of targeted therapies. NICE has played a crucial role in expediting approvals for new agents such as next-generation antiandrogens and radiopharmaceuticals.
The UK is also host to a robust ecosystem of research university institutions and clinical trial networks, which makes it a center for the development of drugs that are used to investigate.
AI integration for cancer diagnostics, especially in pathology and imaging, will go a long way in further improving patient outcomes. FMI opines that the CAGR of the United Kingdom is 9.1% from 2025 to 2035.
Germany is leading the way in CRPC treatment innovation, supported by robust pharmaceutical R&D, excellent clinical standards, and organized health insurance coverage. Precision oncology is a focus area for the country, with molecular profiling informing treatment choice in CRPC patients.
German biotech companies are actively engaged in the development of hormonal agents, radioligand therapies, and immunotherapies against advanced prostate cancers.
The decentralized system of healthcare provides regional innovation, primarily in academic medical centers with advanced clinical trials. Evidence-based medicine and pharmacovigilance in Germany create a high standard of treatment optimization. FMI forecasts that Germany's CAGR will be 9.4% from 2025 to 2035.
South Korea is experiencing rapid growth in CRPC treatments, driven by high healthcare digitization, a technology-driven pharmaceutical industry, and rising public health investments. The government's "Biohealth Industry Vision" seeks to position Korea as a global biopharma hub, which is evident in its growing pipeline of cutting-edge therapeutics.
Hospitals are incorporating AI and machine learning into cancer diagnosis, allowing for early and precise detection of disease progression. South Korean firms are becoming contract manufacturers for international CRPC drug developers and producing indigenous treatments.
In addition, government-sponsored screening programs are increasing the level of early-stage detection, enhancing long-term survival. FMI opines that the CAGR of South Korea is 9.6% from 2025 to 2035.
Japan's CRPC treatment model is defined by its high priority for geriatric care, pharma industry driven by innovation, and heavy use of molecular diagnostics. The Ministry of Health, Labour and Welfare (MHLW) offers accelerated approvals of cancer therapies, allowing prompt industry entry of new therapeutics. Japanese firms are specializing in radiopharmaceuticals and immune-oncology convergence.
The nation is at the forefront of carrying out real-world evidence research, especially with respect to the long-term effects of antiandrogens and radioligand therapies. Precision medicine is directly encouraged through national genomic programs. FMI forecasts that the CAGR of Japan is 9.3% from 2025 to 2035.
France's CRPC treatment industry is growing steadily as a result of a well-balanced combination of public sector support, pharma innovation, and high clinical standards. The national cancer plan in the country encourages early detection, with PSA screening being widely available.
French oncologists are taking advantage of combination regimens and increasingly prescribing targeted therapies on the basis of biomarker profiles. The availability of a vast academic-clinical research environment, supported by EU collaborations, is speeding up trial activity in immunotherapy and hormone-refractory disease.
France is also investigating AI-facilitated diagnostic workflows and laboratory automation for rapid turnaround times. FMI is of the opinion that the CAGR of France is 9.2% from 2025 to 2035.
Italy is investing in regional oncology networks to enhance CRPC treatment access and reduce diagnosis-to-treatment times. The Italian Medicines Agency (AIFA) has simplified its drug evaluation procedures, resulting in quicker reimbursement approvals for new therapies.
Rome, Milan, and Florence oncology centers are performing high-impact research on radioligand therapy and new hormone inhibitors. The focus on public health campaigns for prostate cancer awareness is enhancing early-stage detection. Italian drug companies are collaborating with biotech startups to co-develop targeted treatments, particularly for hormone-refractory instances.
Electronic health record integration is facilitating personalized treatment monitoring and enhanced patient management across geographies. FMI opines that the CAGR of Italy is 9.1% from 2025 to 2035.
Australia and New Zealand remain promising locations for oncology trials, particularly for advanced prostate cancer. Australia's Therapeutic Goods Administration (TGA) has a forward-thinking approach to expediting approvals for life-saving medicines, while New Zealand's PHARMAC system is becoming more supportive of innovative oncology treatments. Early screening programs are common, and the region has one of the highest healthcare quality scores in the world.
CRPC treatment guidelines are becoming more uniform due to national clinical guidelines and tracking of real-world outcomes. FMI projects that the CAGR of both regions is 9.4% from 2025 to 2035.
(Surveyed Q4 2024, n=500 stakeholder participants evenly distributed across oncologists, pharma executives, hospital procurement heads, and regulatory experts in the USA, Western Europe, Japan, and South Korea)
| Countries | Regulatory & Policy Impact |
|---|---|
| USA | FDA breakthrough & fast-track designations streamline drug approvals; CMS value-based cancer care model reforms encourage value-based cancer care models. |
| India | CDSCO regulates approvals, but uncertainty and lack of fast-tracking for oncology products restrain sector entry, and pricing below the NPPA ceiling. |
| China | NMPA reforms facilitate conditional approval of cancer drugs; inclusion in NRDL is pivotal for adoption, with domestic over foreign brands preferred. |
| UK | NICE has high cost-effectiveness thresholds; the Cancer Drug Fund (CDF) allows coverage of expensive treatments under conditional coverage. |
| Germany | AMNOG price law requires early benefit assessment; industry access is promoted, but is contingent on HTA assessments. |
| South Korea | HIRA rigidly uses reimbursement assessment; delayed price negotiations slow down the launch of new agents, especially imported drugs. |
| Japan | PMDA approval is relatively rapid, but twice-yearly drug price revisions and low reimbursement constrain innovation-led product launches. |
| France | HAS and CEPS determine coverage and price; ASMR scores heavily determine reimbursement and industry success. |
| Italy | Regional alignment to achieve full industry access is needed to obtain AIFA approval; MEAs are also commonly employed in the case of expensive CRPC medications. |
| Australia & New Zealand | TGA approval and subsequent consideration by PBAC decide access; reimbursement only for documented survival benefit cases. |
The industry for Castration-Resistant Prostate Cancer (CRPC) treatment is moderately concentrated, with a few large global pharmaceutical players holding sway in the industry.
Leading majors are distinguishing themselves with pricing models, product development, strategic alliances, and entering developing sectors. R&D expenditure is aimed at next-generation medicines, such as radioligand treatments, targeted inhibitors, and enhanced antibody-drug conjugates (ADCs).
AstraZeneca bought Fusion Pharmaceuticals in 2024 for as much as USD 2.4 billion, a step to enhance its radioconjugate therapy in cancer treatment, such as in advanced prostate cancer.
Novartis also took a big step in acquiring Mariana Oncology in May 2024 to fortify its radioligand therapy (RLT) portfolio with a view to addressing unmet needs in advanced oncology, such as prostate cancer.
In the meantime, Johnson & Johnson finalized its acquisition of Ambrx Biopharma in March 2024, gaining control over a pipeline that features ARX517, a PSMA-targeting ADC designed for the treatment of metastatic CRPC.
Johnson & Johnson (32%)
Leads with AR inhibitor Zytiga and Erleada for advanced prostate cancer.
Pfizer/Astellas (28%)
Leads with Xtandi, the most prescribed AR inhibitor in the world.
Bayer (15%)
Disrupted with differentiated AR antagonist Nubeqa with improved safety profile.
Novartis (10%)
Disrupting with Pluvicto, a targeted radiopharmaceutical therapy.
AstraZeneca (8%)
Focusing on precision medicine with PARP inhibitor Lynparza for BRCA+ patients.
Merck & Co. (5%)
Examining combinations of immunotherapies with Keytruda in CRPC.
Hormonal therapy, Chemotherapy, Immunotherapy, Radiotherapy
Antineoplastic, Non-steroidal Antiandrogen, Corticosteroids, Microtubule Inhibitor
Oral Route, Injectable Route
Hospital Pharmacies, Retail Pharmacies, Online Pharmacies
North America, Latin America, Europe, South Asia, East Asia, Oceania, MEA
Advances in radioligand therapies, androgen receptor inhibitors, and novel hormonal agents are driving therapeutic innovation.
Pharmaceuticals are making investments in dual-mechanism therapies and developing combination regimens to maximize efficacy against resistant tumor pathways.
Precision oncology is revolutionizing treatment paradigms by facilitating patient-specific therapies based on genomic and molecular profiling.
Regulatory agencies are streamlining accelerated approval processes, particularly for breakthrough therapies, forcing companies to innovate quickly while ensuring safety compliance.
Asia-Pacific and some Latin American nations are seeing increased adoption owing to increasing incidence, improved diagnostics, and improved access to advanced therapeutics.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 5: Global Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 9: North America Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 10: North America Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 11: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 12: Latin America Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 13: Latin America Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 14: Latin America Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 15: Latin America Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 16: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 17: Europe Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 18: Europe Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 19: Europe Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 20: Europe Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 21: South Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: South Asia Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 23: South Asia Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 24: South Asia Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 25: South Asia Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 26: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 27: East Asia Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 28: East Asia Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 29: East Asia Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 30: East Asia Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 31: Oceania Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 32: Oceania Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 33: Oceania Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 34: Oceania Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 35: Oceania Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Table 36: MEA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 37: MEA Market Value (US$ Million) Forecast by Therapy Type, 2018 to 2033
Table 38: MEA Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 39: MEA Market Value (US$ Million) Forecast by Drug Delivery Method , 2018 to 2033
Table 40: MEA Market Value (US$ Million) Forecast by Distribution Channel, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 4: Global Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 5: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 6: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 7: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 8: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 9: Global Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 10: Global Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 11: Global Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 12: Global Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 13: Global Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 14: Global Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 15: Global Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 16: Global Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 17: Global Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 18: Global Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 19: Global Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 20: Global Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 21: Global Market Attractiveness by Therapy Type, 2023 to 2033
Figure 22: Global Market Attractiveness by Drug Class, 2023 to 2033
Figure 23: Global Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 24: Global Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 25: Global Market Attractiveness by Region, 2023 to 2033
Figure 26: North America Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 27: North America Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 28: North America Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 29: North America Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 30: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 31: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 34: North America Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 35: North America Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 36: North America Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 37: North America Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 38: North America Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 39: North America Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 40: North America Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 41: North America Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 42: North America Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 43: North America Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 44: North America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 45: North America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 46: North America Market Attractiveness by Therapy Type, 2023 to 2033
Figure 47: North America Market Attractiveness by Drug Class, 2023 to 2033
Figure 48: North America Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 49: North America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 50: North America Market Attractiveness by Country, 2023 to 2033
Figure 51: Latin America Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 52: Latin America Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 53: Latin America Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 54: Latin America Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 55: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 56: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 57: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 58: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 59: Latin America Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 60: Latin America Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 61: Latin America Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 62: Latin America Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 63: Latin America Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 64: Latin America Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 65: Latin America Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 66: Latin America Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 67: Latin America Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 68: Latin America Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 69: Latin America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 70: Latin America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 71: Latin America Market Attractiveness by Therapy Type, 2023 to 2033
Figure 72: Latin America Market Attractiveness by Drug Class, 2023 to 2033
Figure 73: Latin America Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 74: Latin America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 75: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 76: Europe Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 77: Europe Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 78: Europe Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 79: Europe Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 80: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 81: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 82: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 83: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 84: Europe Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 85: Europe Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 86: Europe Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 87: Europe Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 88: Europe Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 89: Europe Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 90: Europe Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 91: Europe Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 92: Europe Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 93: Europe Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 94: Europe Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 95: Europe Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 96: Europe Market Attractiveness by Therapy Type, 2023 to 2033
Figure 97: Europe Market Attractiveness by Drug Class, 2023 to 2033
Figure 98: Europe Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 99: Europe Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 100: Europe Market Attractiveness by Country, 2023 to 2033
Figure 101: South Asia Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 102: South Asia Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 103: South Asia Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 104: South Asia Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 105: South Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 106: South Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 107: South Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 108: South Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 109: South Asia Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 110: South Asia Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 111: South Asia Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 112: South Asia Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 113: South Asia Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 114: South Asia Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 115: South Asia Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 116: South Asia Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 117: South Asia Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 118: South Asia Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 119: South Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 120: South Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 121: South Asia Market Attractiveness by Therapy Type, 2023 to 2033
Figure 122: South Asia Market Attractiveness by Drug Class, 2023 to 2033
Figure 123: South Asia Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 124: South Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 125: South Asia Market Attractiveness by Country, 2023 to 2033
Figure 126: East Asia Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 127: East Asia Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 128: East Asia Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 129: East Asia Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 130: East Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 131: East Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 132: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 133: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 134: East Asia Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 135: East Asia Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 136: East Asia Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 137: East Asia Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 138: East Asia Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 139: East Asia Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 140: East Asia Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 141: East Asia Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 142: East Asia Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 143: East Asia Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 144: East Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 145: East Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 146: East Asia Market Attractiveness by Therapy Type, 2023 to 2033
Figure 147: East Asia Market Attractiveness by Drug Class, 2023 to 2033
Figure 148: East Asia Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 149: East Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 150: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 151: Oceania Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 152: Oceania Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 153: Oceania Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 154: Oceania Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 155: Oceania Market Value (US$ Million) by Country, 2023 to 2033
Figure 156: Oceania Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 157: Oceania Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 158: Oceania Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 159: Oceania Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 160: Oceania Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 161: Oceania Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 162: Oceania Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 163: Oceania Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 164: Oceania Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 165: Oceania Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 166: Oceania Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 167: Oceania Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 168: Oceania Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 169: Oceania Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 170: Oceania Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 171: Oceania Market Attractiveness by Therapy Type, 2023 to 2033
Figure 172: Oceania Market Attractiveness by Drug Class, 2023 to 2033
Figure 173: Oceania Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 174: Oceania Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 175: Oceania Market Attractiveness by Country, 2023 to 2033
Figure 176: MEA Market Value (US$ Million) by Therapy Type, 2023 to 2033
Figure 177: MEA Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 178: MEA Market Value (US$ Million) by Drug Delivery Method , 2023 to 2033
Figure 179: MEA Market Value (US$ Million) by Distribution Channel, 2023 to 2033
Figure 180: MEA Market Value (US$ Million) by Country, 2023 to 2033
Figure 181: MEA Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 182: MEA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 183: MEA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 184: MEA Market Value (US$ Million) Analysis by Therapy Type, 2018 to 2033
Figure 185: MEA Market Value Share (%) and BPS Analysis by Therapy Type, 2023 to 2033
Figure 186: MEA Market Y-o-Y Growth (%) Projections by Therapy Type, 2023 to 2033
Figure 187: MEA Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 188: MEA Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 189: MEA Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 190: MEA Market Value (US$ Million) Analysis by Drug Delivery Method , 2018 to 2033
Figure 191: MEA Market Value Share (%) and BPS Analysis by Drug Delivery Method , 2023 to 2033
Figure 192: MEA Market Y-o-Y Growth (%) Projections by Drug Delivery Method , 2023 to 2033
Figure 193: MEA Market Value (US$ Million) Analysis by Distribution Channel, 2018 to 2033
Figure 194: MEA Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 195: MEA Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 196: MEA Market Attractiveness by Therapy Type, 2023 to 2033
Figure 197: MEA Market Attractiveness by Drug Class, 2023 to 2033
Figure 198: MEA Market Attractiveness by Drug Delivery Method , 2023 to 2033
Figure 199: MEA Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 200: MEA Market Attractiveness by Country, 2023 to 2033






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Prostate-Specific Antigen Testing Market Analysis - Size, Share & Forecast 2025 to 2035
Prostate Health Market Trends - Growth, Demand & Forecast 2025 to 2035
Prostate Cancer Market Growth - Trends & Forecast 2025 to 2035
Breast & Prostate Cancer Diagnostics Market in Europe – Trends & Forecast 2025 to 2035
Hormone Sensitive Prostate Cancer Market – Trends & Forecast 2025 to 2035
Hormone Sensitive Advanced Prostate Cancer Treatment Market Size and Share Forecast Outlook 2025 to 2035
Benign Prostatic Hyperplasia (BPH) Prostate Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market
Water Treatment System Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Chemical Market Size and Share Forecast Outlook 2025 to 2035
Algae Treatment Chemical Market Forecast and Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA