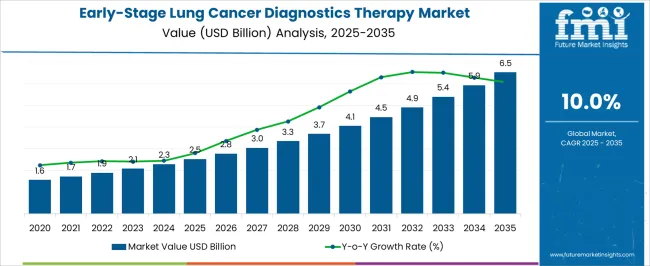
The Early-Stage Lung Cancer Diagnostics Therapy Market is estimated to be valued at USD 2.5 billion in 2025 and is projected to reach USD 6.5 billion by 2035, registering a compound annual growth rate (CAGR) of 10.0% over the forecast period.
| Metric | Value |
|---|---|
| Early-Stage Lung Cancer Diagnostics Therapy Market Estimated Value in (2025 E) | USD 2.5 billion |
| Early-Stage Lung Cancer Diagnostics Therapy Market Forecast Value in (2035 F) | USD 6.5 billion |
| Forecast CAGR (2025 to 2035) | 10.0% |
The Early-Stage Lung Cancer Diagnostics Therapy market is experiencing robust growth driven by the increasing prevalence of lung cancer and the rising emphasis on early detection to improve patient outcomes. The current market scenario is shaped by the adoption of precision diagnostics that enable targeted therapeutic interventions, with growing investments in healthcare infrastructure supporting the expansion. Advanced molecular testing techniques and biomarker-based diagnostics have enhanced the accuracy and efficiency of early-stage detection, facilitating timely treatment decisions.
Rising awareness among healthcare professionals and patients about the benefits of early diagnosis is further contributing to market adoption. Additionally, integration of diagnostic tools into routine clinical workflows, alongside increasing demand for minimally invasive procedures, is paving the way for market growth.
The future outlook is favorable, as healthcare systems continue to prioritize screening programs and the development of companion diagnostics that guide personalized treatment The convergence of technological innovation, patient-centered care, and regulatory support is expected to reinforce the market trajectory, positioning early-stage lung cancer diagnostics as a critical component of modern oncology care.
The early-stage lung cancer diagnostics therapy market is segmented by cell type, test type, end user, and geographic regions. By cell type, early-stage lung cancer diagnostics therapy market is divided into Non Small-Cell Lung Cancer and Small-Cell Lung Cancer. In terms of test type, early-stage lung cancer diagnostics therapy market is classified into EGFR Test, CA Test, BRAF Test, ALK Test, ROS1 Test, HER2 Test, RET Test, and Others. Based on end user, early-stage lung cancer diagnostics therapy market is segmented into Hospital Associated Laboratories, Cancer Research Laboratories, and Independent Cancer Diagnostic Laboratories. Regionally, the early-stage lung cancer diagnostics therapy industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
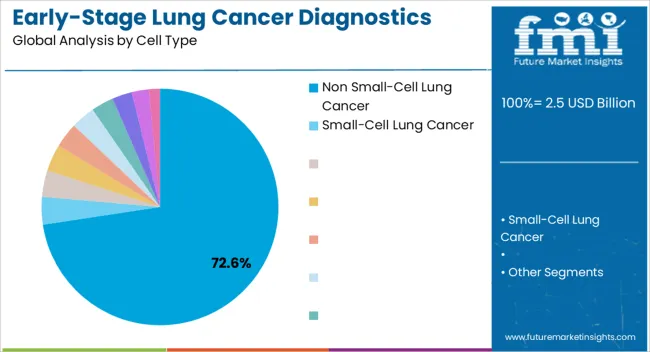
The non small-cell lung cancer cell type is projected to hold 72.60% of the market revenue share in 2025, establishing it as the leading cell type segment. This dominance has been facilitated by the high prevalence of non small-cell lung cancer cases globally, which accounts for the majority of lung cancer diagnoses. Growth in this segment has been driven by advancements in molecular characterization and biomarker identification, enabling precise detection and monitoring of early-stage disease.
Adoption has been supported by clinicians prioritizing early intervention strategies to improve survival rates and reduce treatment costs. The segment has benefitted from enhanced sensitivity and specificity of diagnostic assays tailored for non small-cell variants, ensuring timely therapeutic decisions.
Moreover, the increased availability of testing infrastructure and the growing emphasis on integrating diagnostics into routine oncology practice have reinforced the adoption of non small-cell lung cancer diagnostics As personalized medicine continues to gain traction, the demand for targeted early-stage diagnostics in this segment is expected to sustain market leadership, offering actionable insights to guide treatment planning and patient management.
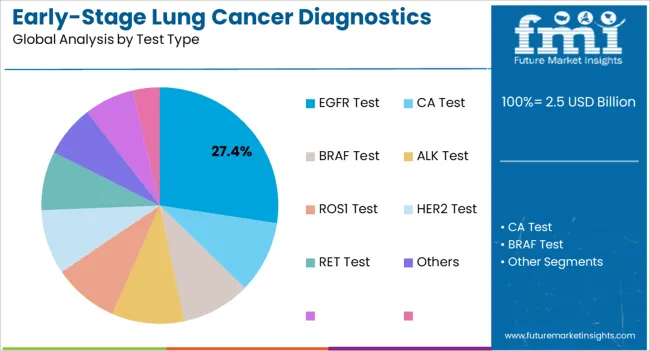
The EGFR test type is anticipated to account for 27.40% of the market revenue share in 2025, emerging as the leading diagnostic test segment. This prominence is being driven by the critical role of EGFR mutation detection in guiding targeted therapy selection, improving treatment efficacy, and optimizing patient outcomes. Adoption has been supported by advancements in molecular diagnostic technologies, which enable highly sensitive and non-invasive testing suitable for early-stage detection.
The segment’s growth has been reinforced by the increasing prevalence of EGFR-positive lung cancer cases and the proven impact of targeted therapies in improving survival rates. Integration of EGFR testing into routine diagnostic workflows has allowed healthcare providers to streamline decision-making and reduce time to treatment initiation.
Additionally, the segment has benefited from rising awareness among oncologists and patients regarding the importance of biomarker-driven therapy selection Future growth is expected to be bolstered by continuous technological innovation, expanded testing coverage, and growing adoption of personalized treatment strategies across oncology centers.
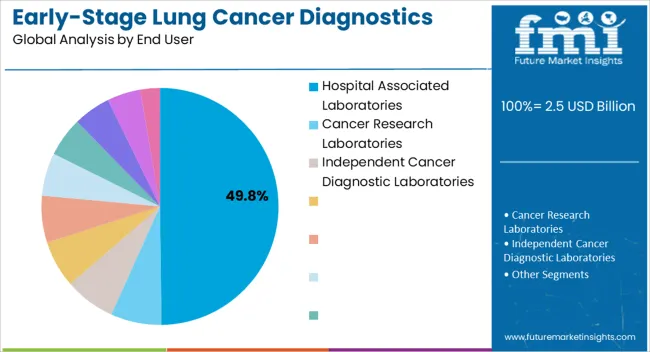
The hospital associated laboratories end-use segment is projected to hold 49.80% of the market revenue share in 2025, positioning it as the largest end-user segment. This leadership is being driven by the central role of hospital laboratories in performing advanced diagnostic testing and facilitating early detection of lung cancer. Adoption has been accelerated by the ability of hospital-associated facilities to integrate high-throughput testing technologies and maintain quality control standards, ensuring accurate and reliable results.
Growth has also been influenced by increasing hospital investments in molecular diagnostic infrastructure and the deployment of specialized oncology units capable of handling complex testing protocols. The segment has benefitted from the trend of consolidating diagnostic services within hospital settings to enable faster reporting, streamlined patient management, and improved clinical decision-making.
Additionally, hospital laboratories are often preferred for their accessibility, professional expertise, and established workflows, which support large-scale testing programs The ongoing expansion of hospital diagnostic services and focus on personalized treatment approaches is expected to sustain the segment’s market leadership in early-stage lung cancer diagnostics.
Cancer is the second most active disease in the world currently by the no. of deaths it is responsible for. Today’s complex time has issued an alarming situation for the consumers as well as the manufacturers to limit the cases with early diagnosis on every term. Early-stage lung cancer diagnostics therapy is performed to diagnose the presence of cancer cells in lungs with immediate care offered. Cancer was a leading cause of death worldwide. According to the National Cancer Institute at the National Institutes of Health, around 8.2 million cancer deaths and 14 million new cases were registered in 2025.
The need for a well-organized early-stage lung cancer diagnostics therapy in is due to the fact that only 18% of the lung cancer cases get recognized while the remaining undiagnosed cancer cases progress into more hostile forms of cancer cells, which reduces the survival rate to only few percent’s in patients that are diagnosed with late-stage lung cancer.
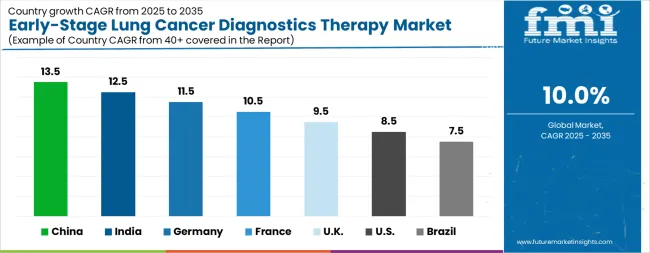
| Country | CAGR |
|---|---|
| China | 13.5% |
| India | 12.5% |
| Germany | 11.5% |
| France | 10.5% |
| UK | 9.5% |
| USA | 8.5% |
| Brazil | 7.5% |
The Early-Stage Lung Cancer Diagnostics Therapy Market is expected to register a CAGR of 10.0% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 13.5%, followed by India at 12.5%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 7.5%, yet still underscores a broadly positive trajectory for the global Early-Stage Lung Cancer Diagnostics Therapy Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 11.5%. The USA Early-Stage Lung Cancer Diagnostics Therapy Market is estimated to be valued at USD 863.3 million in 2025 and is anticipated to reach a valuation of USD 2.0 billion by 2035. Sales are projected to rise at a CAGR of 8.5% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 136.5 million and USD 69.8 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 2.5 Billion |
| Cell Type | Non Small-Cell Lung Cancer and Small-Cell Lung Cancer |
| Test Type | EGFR Test, CA Test, BRAF Test, ALK Test, ROS1 Test, HER2 Test, RET Test, and Others |
| End User | Hospital Associated Laboratories, Cancer Research Laboratories, and Independent Cancer Diagnostic Laboratories |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
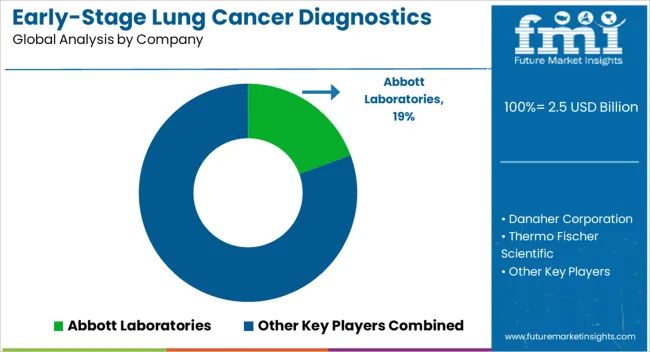
| Key Companies Profiled | Abbott Laboratories, Danaher Corporation, Thermo Fischer Scientific, QIAGEN N.V, Illumina, Inc., Quest Diagnostics Incorporated, NanoString Technologies, and NeoGenomics Laboratories |
The global early-stage lung cancer diagnostics therapy market is estimated to be valued at USD 2.5 billion in 2025.
The market size for the early-stage lung cancer diagnostics therapy market is projected to reach USD 6.5 billion by 2035.
The early-stage lung cancer diagnostics therapy market is expected to grow at a 10.0% CAGR between 2025 and 2035.
The key product types in early-stage lung cancer diagnostics therapy market are non small-cell lung cancer and small-cell lung cancer.
In terms of test type, egfr test segment to command 27.4% share in the early-stage lung cancer diagnostics therapy market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Lung Biopsy Systems Market Size and Share Forecast Outlook 2025 to 2035
The lung disease therapeutics market is segmented by disease type, treatment type and distribution channel from 2025 to 2035
Lung Cancer Surgery Market - Size, Share, and Forecast 2025 to 2035
Lung Cancer Therapeutics Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
Lung Cancer PCR Panel Market Trends, Growth, Demand & Forecast 2025 to 2035
Lung Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Plunger Stopper Market Insights – Trends & Growth Forecast 2024-2034
Cold Plunge Tub Market Analysis by Growth, Trends and Forecast from 2025 to 2035
Robotic Lung Biopsy Market Size and Share Forecast Outlook 2025 to 2035
Small Cell Lung Cancer (SCLC) Treatment Market Size and Share Forecast Outlook 2025 to 2035
Interstitial Lung Disease Treatment Market
Non-Small Cell Lung Carcinoma (NSCLC) Market Size and Share Forecast Outlook 2025 to 2035
Non-Small Cell Lung Cancer Market Size and Share Forecast Outlook 2025 to 2035
PD1 Non-Small Cell Lung Cancer Treatment Market - Growth & Outlook 2025 to 2035
Progressive Fibrosing Interstitial Lung Disease (PF-ILD) Treatment Market – Growth & Future Outlook 2025 to 2035
IV Therapy and Vein Access Devices Market Insights – Trends & Forecast 2024-2034
Mesotherapy Market Size and Share Forecast Outlook 2025 to 2035
Cryotherapy Market Growth - Demand, Trends & Emerging Applications 2025 to 2035
Aromatherapy Market Size and Share Forecast Outlook 2025 to 2035
Radiotherapy Positioning Devices Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA