The Endoscope Procedure Kits Market is estimated to be valued at USD 16.5 billion in 2025 and is projected to reach USD 40.5 billion by 2035, registering a compound annual growth rate (CAGR) of 9.4% over the forecast period.
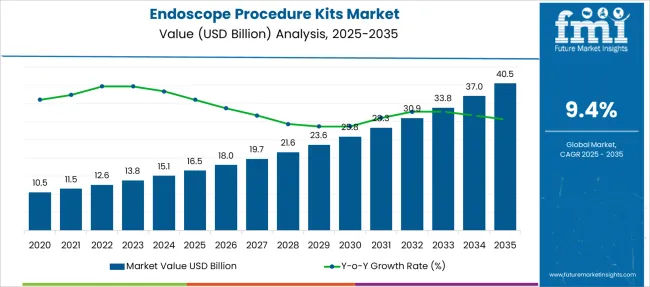
| Metric | Value |
|---|---|
| Endoscope Procedure Kits Market Estimated Value in (2025 E) | USD 16.5 billion |
| Endoscope Procedure Kits Market Forecast Value in (2035 F) | USD 40.5 billion |
| Forecast CAGR (2025 to 2035) | 9.4% |
The endoscope procedure kits market is expanding steadily due to the increasing global volume of endoscopic procedures and the need for standardized infection prevention practices. The growing emphasis on reducing hospital acquired infections has led healthcare providers to adopt procedure specific kits that improve workflow efficiency and regulatory compliance.
These kits streamline the cleaning and handling of endoscopes while minimizing human error and cross contamination risks. Technological advancements in kit design, such as single use components and improved pre cleaning agents, have further reinforced demand.
With rising gastrointestinal and pulmonary diagnostic procedures, the demand for reliable and ready to use endoscope kits is surging. Future growth will be supported by hospital investments in infection control infrastructure, rising outpatient endoscopy volumes, and ongoing efforts to meet stringent sterilization guidelines across developed and emerging healthcare markets.
The market is segmented by Type, Application, and End Users and region. By Type, the market is divided into Bedside Pre-Cleaning Kits, Transport Pads, Gauze Pads, Suction Tubing, Lubricating Jelly, Endoscope Valves System, Pre-cleaning Valve Kit, Brush Kit, and Others. In terms of Application, the market is classified into Gastrointestinal Endoscopy, Bronchoscopy, Arthroscopy, Laparoscopy, Urology, and Others. Based on End Users, the market is segmented into Hospitals and Clinics, Ambulatory Surgical Centers (ASCs), Diagnostic Centers, and Others. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
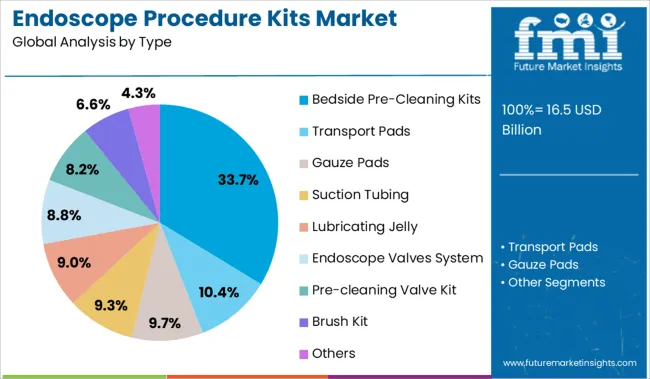
The bedside pre cleaning kits segment is expected to hold 33.70% of the total revenue share by 2025 within the type category, positioning it as the most dominant type. This growth is being fueled by the critical role these kits play in the initial decontamination phase, where prompt and proper cleaning significantly reduces the risk of biofilm formation and contamination.
These kits allow for immediate bedside application, minimizing the time between use and reprocessing and ensuring compliance with infection prevention standards. Hospitals and clinics are increasingly standardizing bedside cleaning protocols, making these kits a key element of operational workflows.
The convenience, effectiveness, and alignment with global reprocessing guidelines have strengthened the position of bedside pre cleaning kits within the type segment.
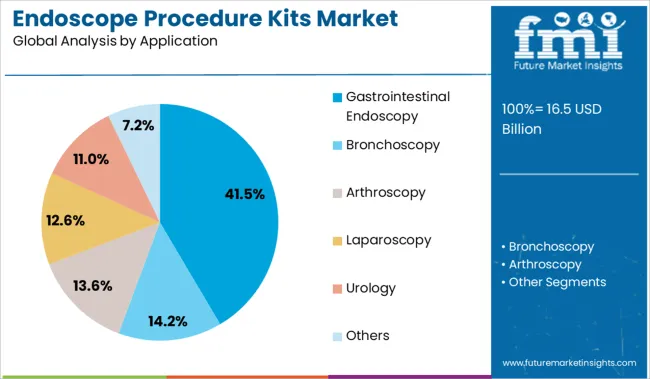
The gastrointestinal endoscopy segment is projected to account for 41.50% of total market revenue by 2025 in the application category, making it the leading segment. This is due to the rising incidence of gastrointestinal disorders and the growing preference for minimally invasive diagnostic procedures.
The complexity and frequency of these procedures necessitate strict adherence to endoscope cleaning and disinfection protocols, increasing the reliance on dedicated procedure kits. These kits support endoscopic workflow standardization and reduce turnaround time between procedures, enhancing operational efficiency in busy gastroenterology units.
As gastrointestinal screenings become more common in preventive healthcare, the demand for reliable and safe procedure kits continues to grow, securing this segment’s dominance.
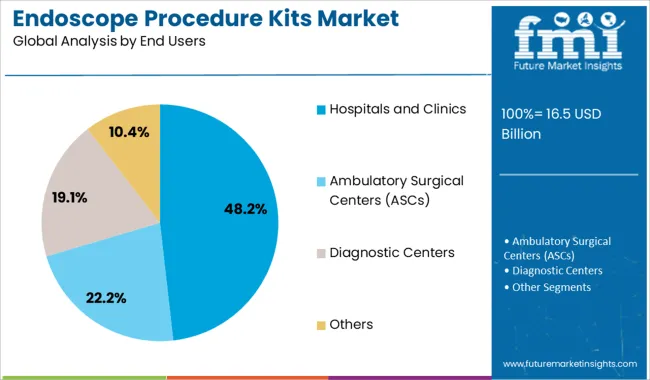
The hospitals and clinics segment is expected to capture 48.20% of the total revenue by 2025 under the end users category, maintaining its position as the largest segment. This dominance is driven by the high volume of endoscopic procedures conducted in institutional healthcare settings and the strict regulatory oversight regarding infection control.
Hospitals and clinics are prioritizing standardized kit usage to maintain consistent reprocessing practices, reduce infection risks, and ensure compliance with health authority protocols. Centralized procurement systems and greater budget allocations for infection prevention in hospitals further support widespread adoption.
With increasing procedural demand and a strong focus on patient safety, hospitals and clinics remain the primary adopters of endoscope procedure kits across global markets.
A fast paced modern lifestyle has led to a variety of gastrointestinal disorders like Gastrointestinal Reflux Disease (GERD), Crohn’s disease, Irritable bowel syndrome (IBS), gastroenteritis, dyspepsia, gallstones and also various forms of Cancer such as Colon Cancer.
This affects not only the aged population, but also the young and upwardly mobile. Hence, it can be reasonably assumed to drive the endoscope procedure kit market demand for the foreseeable future. There is also a growing preference for minimally invasive non-surgical procedures with diagnostic and therapeutic application.
An endoscopy does not require a patient to stay in the hospital for very long (if at all) which brings down the stress levels and associated costs of the treatment.Greater patient awareness and technological innovation are also primary reasons that the endoscope procedure kit market is growing exponentially. Another factor is the reimbursement option available for preventive screening techniques like various endoscopies.
The American Society of Gastrointestinal Endoscopy estimates the average Adenoma Detection Rate (ADR) to be around 25%. This also requires the use of endoscope procedure kit, further boosting growth. The USA FDA policies are also encouraging and support quick approval of these devices.
There are a few factors which restrain the endoscope procedure kit market from reaching its true potential, the availability of advanced alternatives being one of them. These devices are very costly and there are limited funds available with governments in developing countries to reimburse hospitals and medical practitioners for their sizeable investment in them.
A lack of awareness on the part of the general public in these countries about the cost-benefit equation of such treatments is also something which must be mentioned.
The main regions served by the endoscope procedure kit market are as follows - North America, Europe, Asia Pacific and the rest of the world (Latin America, Middle East and Africa). North America is the largest market at present followed by Europe.
This is mainly due to the availability of advanced healthcare facilities, an aging population, strong government support in the form of social security benefits and an educated and aware populace.
Asia Pacific can be expected to rapidly catch up to the West in the medium to long term for demanding endoscope procedure kits because of greater investment in healthcare, rising prevalence of gastroenteritis disorders and lastly a growing awareness of such procedures.
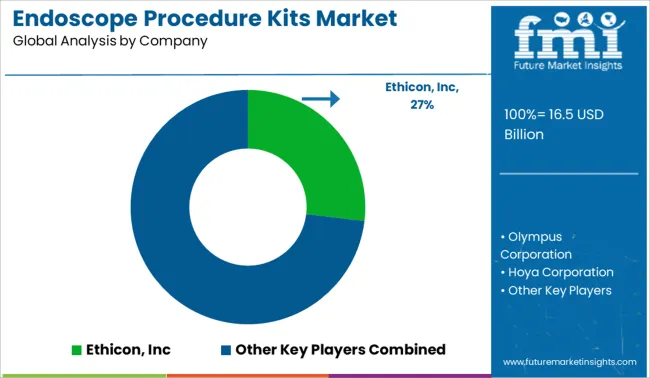
The endoscope procedure kit market key players include Ethicon, Inc. (USA), Olympus Corporation (Japan), Hoya Corporation (Japan), Given Imaging Ltd. (Israel), Minntech Corporation (USA) Fujifilm Holding Corporation (Japan), Advanced Sterilization Products Services Inc. (USA), Steris Corporation (USA),Boston Scientific Corporation (USA), Pentax Medical Corporation (Japan), Stryker Corporation (USA). KARL STORZ GmBH (Germany), Smith & Nephew plc (UK), Medtronic plc (Ireland), Richard Wolf GmBH (Germany), CONMED Corporation (USA), and Cook Medical Incorporated (USA).
The research report presents a comprehensive assessment of the market and contains thoughtful insights, facts, historical data, and statistically supported and industry-validated market data. It also contains projections using a suitable set of assumptions and methodologies.
The research report provides analysis and information according to market segments such as geographies, types and applications.
The global endoscope procedure kits market is estimated to be valued at USD 16.5 billion in 2025.
The market size for the endoscope procedure kits market is projected to reach USD 40.5 billion by 2035.
The endoscope procedure kits market is expected to grow at a 9.4% CAGR between 2025 and 2035.
The key product types in endoscope procedure kits market are bedside pre-cleaning kits, transport pads, gauze pads, suction tubing, lubricating jelly, endoscope valves system, pre-cleaning valve kit, brush kit and others.
In terms of application, gastrointestinal endoscopy segment to command 41.5% share in the endoscope procedure kits market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Endoscope Protective Barrier Covers Market Size and Share Forecast Outlook 2025 to 2035
Endoscope Reprocessing Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Endoscope Detergents And Disinfectants Market Size and Share Forecast Outlook 2025 to 2035
Endoscope Reprocessing Device Market – Trends & Forecast 2025-2035
Endoscope Tracking Solutions Market
Endoscope Leak Detection Device Market
Sialendoscopes Market Size and Share Forecast Outlook 2025 to 2035
Capsule Endoscope and Workstations Market - Growth & Demand 2025 to 2035
Flexible Endoscopes Market Growth - Trends & Forecast 2025 to 2035
Disposable Endoscopes Market Insights – Size, Share & Forecast 2025 to 2035
Veterinary Endoscopes Market Insights - Trends, Applications & Forecast 2024 to 2034
Chip-on-the-Tip Endoscopes Market Size and Share Forecast Outlook 2025 to 2035
Veterinary Micro-fibre Endoscope Market Size and Share Forecast Outlook 2025 to 2035
Medical Procedure Tray Market
The Veterinary Procedure Lights Market is segmented by Product, Modality and End User from 2025 to 2035
The Arthroscopy Procedure and Products Market is segmented by product type, application, and end user from 2025 to 2035
Hysteroscopy Procedures Market Size and Share Forecast Outlook 2025 to 2035
Wrist Fixator Procedure Kit Market
Surgical Robot Procedures Market Size and Share Forecast Outlook 2025 to 2035
Laparoscopic Gynecological Procedures Market – Growth & Trends 2024-2034

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA