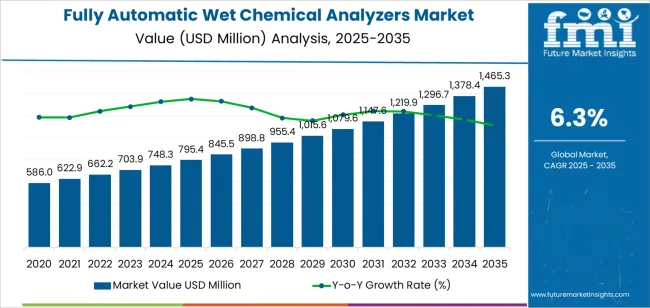
The global fully automatic wet chemical analyzers market is projected to grow from USD 795.4 million in 2025 to approximately USD 1465.2 million by 2035, recording an absolute increase of USD 669.8 million over the forecast period. This translates into a total growth of 84.2%, with the market forecast to expand at a compound annual growth rate (CAGR) of 6.3% between 2025 and 2035. The overall market size is expected to grow by 1.8X during the same period, supported by increasing demand from pharmaceutical quality control operations, expanding food safety testing requirements across manufacturing facilities, and growing adoption of automated analytical systems requiring precise chemical analysis capabilities in laboratory and industrial process monitoring applications.
The fully automatic wet chemical analyzers sector demonstrates robust expansion patterns driven by stringent regulatory compliance requirements, particularly in pharmaceutical manufacturing where analytical validation protocols mandate automated testing systems. Food and beverage quality assurance operations provide substantial demand channels, with contamination detection requirements and nutritional analysis specifications supporting premium analyzer adoption. Laboratory modernization investments across research institutions and industrial facilities create procurement opportunities, while process monitoring applications in chemical manufacturing and environmental testing maintain consistent equipment replacement cycles.
Technical performance characteristics position fully automatic wet chemical analyzers as essential instruments in quality control and process monitoring operations. These systems deliver analytical precision, sample throughput efficiency, and data integrity benefits that manual testing methods cannot match. Specifications for detection limits, measurement accuracy, and automation capabilities establish market entry requirements that favor established manufacturers with comprehensive validation documentation. Supply chain relationships between analyzer producers and pharmaceutical companies create switching costs through method validation requirements, regulatory filing dependencies, and technical support commitments.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 795.4 million |
| Market Forecast Value (2035) | USD 1465.2 million |
| Forecast CAGR (2025-2035) | 6.3% |
| REGULATORY COMPLIANCE REQUIREMENTS | ANALYTICAL EFFICIENCY DEMANDS | QUALITY ASSURANCE STANDARDS |
|---|---|---|
| Pharmaceutical Manufacturing Standards | Laboratory Automation Requirements | Data Integrity Standards |
| Continuous expansion of pharmaceutical production across regulated markets driving sustained demand for validated analytical instruments. | Modern laboratory operations require automated systems delivering precise analytical control and enhanced sample throughput efficiency. | Regulatory requirements establishing performance benchmarks favoring high-quality automated analytical equipment. |
| Food Safety Regulations | Process Monitoring Applications | Method Validation Requirements |
| Growing food safety enforcement and quality testing mandates creating sustained analyzer demand supporting compliance operations. | Chemical manufacturers and industrial processors investing in automated monitoring systems offering consistent performance while maintaining operational efficiency. | Quality standards requiring superior analytical precision and resistance to measurement variability in critical applications. |
| Environmental Testing Standards | Operational Efficiency Focus | Audit Trail Compliance |
| Increasing environmental monitoring requirements and water quality testing mandates requiring advanced analytical capabilities supporting regulatory reporting. | Testing laboratories with proven automation capabilities required for high-throughput analytical applications. | Diverse regulatory requirements and documentation standards driving need for sophisticated automated systems. |
| Category | Segments Covered |
|---|---|
| By Analyzer Type | Automated Discrete Analyzers, Continuous Flow Analyzers |
| By Application | Food and Beverage, Pharmaceutical, Laboratory, Others |
| By Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
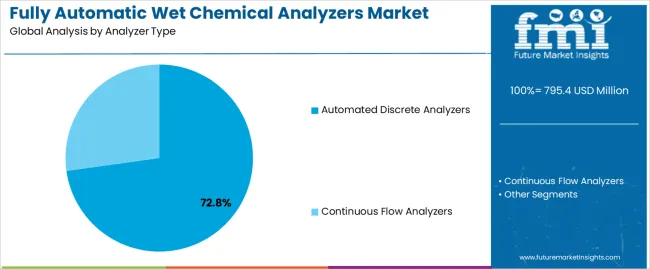
| Segment | 2025 to 2035 Outlook |
|---|---|
| Automated Discrete Analyzers |
|
| Continuous Flow Analyzers |
|
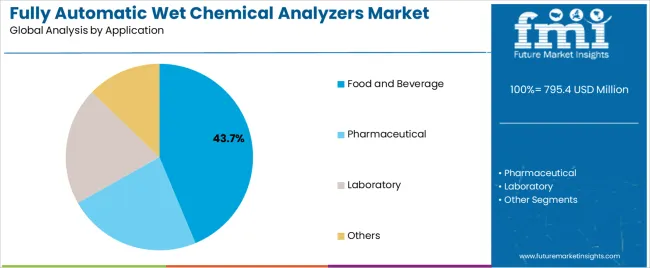
| Segment | 2025 to 2035 Outlook |
|---|---|
| Food and Beverage |
|
| Pharmaceutical |
|
| Laboratory |
|
| Others |
|
| DRIVERS | RESTRAINTS | KEY TRENDS |
|---|---|---|
| Pharmaceutical Manufacturing Growth | High Capital Investment | Automation Integration |
| Continuous expansion of pharmaceutical production capacity across emerging markets driving sustained analyzer demand for quality control operations. | Initial equipment costs and installation expenses affecting adoption rates among smaller laboratories and testing facilities. | Integration of advanced automation systems, robotic sample handling, and data management innovations enabling superior operational efficiency. |
| Food Safety Enforcement | Maintenance Complexity | Method Standardization |
| Increasing food safety regulations and testing mandates creating demand for validated analytical systems supporting compliance operations. | Technical expertise requirements and consumable costs increasing total ownership expenses and operational complexity. | Development of standardized analytical methods and harmonized testing protocols providing enhanced regulatory acceptance. |
| Environmental Monitoring | Alternative Technologies | Digital Connectivity |
| Growing water quality testing requirements and environmental compliance programs creating opportunities for automated analytical solutions. | Competition from spectroscopic techniques and rapid testing methods presenting competitive pressure in cost-sensitive applications. | Enhanced data connectivity between analyzers and laboratory information management systems providing integrated workflow capabilities. |
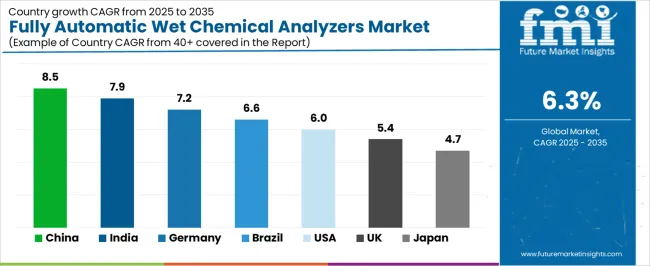
| Country | CAGR (2025-2035) |
|---|---|
| China | 8.5% |
| India | 7.9% |
| Germany | 7.2% |
| Brazil | 6.6% |
| USA | 6% |
| UK | 5.4% |
| Japan | 4.7% |
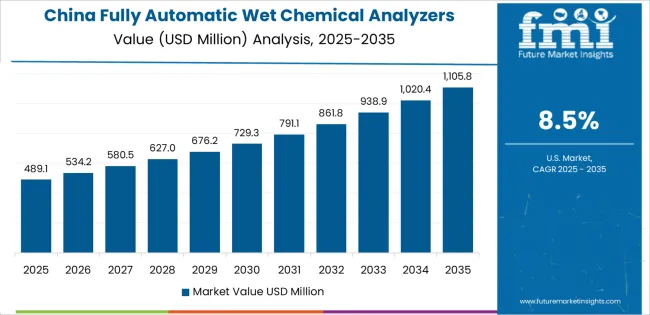
Revenue from fully automatic wet chemical analyzers in China is projected to exhibit strong growth with a market value of USD 286.7 million by 2035, driven by expanding pharmaceutical manufacturing infrastructure and comprehensive food safety testing system development creating substantial opportunities for analyzer suppliers across quality control operations, environmental monitoring applications, and research laboratory sectors. The country's pharmaceutical production leadership and expanding food processing capabilities are creating significant demand for both routine and specialized analytical instruments. Major pharmaceutical manufacturers and food companies are establishing comprehensive testing laboratories to support large-scale production operations and meet growing domestic and export quality standards requiring validated analytical systems.
Pharmaceutical manufacturing modernization programs are supporting widespread adoption of automated analytical systems across quality control laboratories, driving demand for high-performance testing instruments. Food safety testing expansion and environmental monitoring program growth are creating substantial opportunities for analyzer suppliers requiring reliable performance and regulatory compliance capabilities. Export-oriented pharmaceutical production and international quality standard implementation are facilitating adoption of premium analytical instruments throughout major manufacturing regions.
Revenue from fully automatic wet chemical analyzers in India is expanding to reach USD 172.8 million by 2035, supported by extensive pharmaceutical manufacturing capacity additions and comprehensive food testing infrastructure development creating sustained demand for reliable analytical instruments across diverse laboratory categories and specialized testing segments. The country's generic pharmaceutical production leadership and expanding food processing sector are driving demand for analyzer solutions that provide consistent analytical performance while supporting cost-effective testing requirements. Pharmaceutical manufacturers and food testing laboratories are investing in automated systems to support growing production operations and regulatory compliance mandates.
Pharmaceutical sector growth and generic drug manufacturing expansion are creating opportunities for analytical instruments across diverse testing segments requiring reliable performance and competitive operational costs. Testing infrastructure development and quality standard advancement are driving investments in analyzer supply chains supporting technical requirements throughout major pharmaceutical manufacturing regions. Food safety testing growth and contract laboratory development programs are enhancing demand for automated analytical systems throughout major industrial centers.
Demand for fully automatic wet chemical analyzers in Germany is projected to reach USD 23.6 million by 2035, supported by the country's leadership in pharmaceutical manufacturing and advanced analytical technologies requiring sophisticated automated systems for drug development and quality control applications. German pharmaceutical manufacturers and testing laboratories are implementing high-quality analytical systems that support advanced validation protocols, operational efficiency, and comprehensive regulatory compliance. The market is characterized by focus on analytical precision, data integrity, and adherence to stringent pharmaceutical and food safety standards.
Pharmaceutical manufacturing investments are prioritizing advanced analytical systems that demonstrate superior measurement accuracy and reliability while meeting German regulatory and quality standards. Technical excellence programs and method validation initiatives are driving adoption of precision-engineered analytical instruments that support advanced quality control systems and regulatory requirements. Research and development programs for pharmaceutical innovation are facilitating adoption of specialized analytical capabilities throughout major pharmaceutical and research centers.
Revenue from fully automatic wet chemical analyzers in Brazil is growing to reach USD 124.4 million by 2035, driven by pharmaceutical manufacturing development programs and increasing food safety testing capacity creating sustained opportunities for analyzer suppliers serving both domestic production operations and export quality verification. The country's expanding pharmaceutical manufacturing base and growing food processing sector are creating demand for analytical instruments that support diverse testing specifications while maintaining performance standards. Testing laboratories and quality control facilities are developing procurement strategies to support operational efficiency and regulatory compliance.
Pharmaceutical manufacturing modernization programs and generic drug production expansion are facilitating adoption of automated analyzers capable of supporting diverse analytical requirements and competitive operational structures. Food safety testing development and environmental monitoring programs are enhancing demand for analytical instruments that support operational efficiency and measurement reliability. Domestic testing capacity additions and regulatory compliance initiatives are creating opportunities for local analytical service capabilities.
Demand for fully automatic wet chemical analyzers in USA is projected to reach USD 37.3 million by 2035, driven by pharmaceutical research excellence and specialty testing capabilities supporting advanced drug development applications and comprehensive quality verification requirements. The country's established pharmaceutical industry and specialized analytical testing sectors are creating demand for high-quality automated systems that support operational performance and regulatory standards. Analyzer manufacturers and scientific equipment suppliers are maintaining comprehensive technical capabilities to support diverse testing requirements.
Pharmaceutical development and specialty chemical testing programs are supporting demand for advanced analyzers that meet rigorous performance and validation standards. Biotechnology research and environmental testing programs are creating opportunities for specialized instruments that provide comprehensive analytical support. Laboratory automation and quality enhancement programs are facilitating adoption of advanced analytical capabilities throughout major pharmaceutical and research facilities.
Revenue from fully automatic wet chemical analyzers in UK is growing to reach USD 86 million by 2035, supported by pharmaceutical research operations and specialized testing laboratory capabilities serving drug development and quality control applications. Post-Brexit regulatory framework adjustments have created opportunities for domestic testing capacity while maintaining analytical method standards required by pharmaceutical manufacturers. Technical expertise in pharmaceutical analysis supports demand for advanced analytical instruments.
Research laboratory operations and pharmaceutical development activities drive consistent analyzer consumption, with contract research organizations and academic institutions providing stable demand channels. Regulatory compliance requirements and data integrity standards favor established analyzer manufacturers with comprehensive validation documentation and technical support capabilities. Laboratory consolidation trends concentrate purchasing decisions with larger operations while creating opportunities for specialized analytical solutions.
Demand for fully automatic wet chemical analyzers in Japan is projected to reach USD 269.6 million by 2035, expanding at a CAGR of 4.7%, driven by pharmaceutical manufacturing excellence and precision analytical capabilities supporting advanced quality standards and comprehensive regulatory applications. The country's established pharmaceutical tradition and stringent analytical requirements are creating demand for high-specification automated systems that support operational performance and regulatory compliance. Analyzer manufacturers maintain comprehensive quality capabilities to support exacting testing requirements.
Pharmaceutical manufacturing and precision analytical testing programs are supporting demand for automated systems that meet rigorous accuracy and reliability standards. Quality assurance protocols and method validation requirements are creating opportunities for specialized instruments that provide comprehensive testing support. Precision measurement and regulatory compliance programs are facilitating adoption of advanced analytical capabilities throughout major pharmaceutical and research facilities.
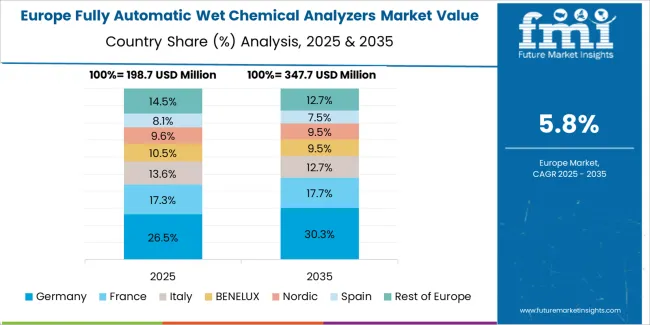
The fully automatic wet chemical analyzers market in Europe is projected to grow from USD 156.2 million in 2025 to USD 269.4 million by 2035, registering a CAGR of 5.6% over the forecast period. Germany is expected to maintain its leadership position with a 31.4% market share in 2025, declining slightly to 30.8% by 2035, supported by its advanced pharmaceutical manufacturing base and major analytical instrument production facilities including established quality control laboratories.
France follows with a 18.2% share in 2025, projected to reach 18.6% by 2035, driven by comprehensive pharmaceutical production operations and food testing laboratory capabilities at major research and industrial centers. The United Kingdom holds a 15.8% share in 2025, expected to decrease to 15.3% by 2035 due to post-Brexit regulatory adjustments and pharmaceutical manufacturing relocations. Italy commands a 13.6% share, while Spain accounts for 11.2% in 2025. The Rest of Europe region is anticipated to gain momentum, expanding its collective share from 9.8% to 10.9% by 2035, attributed to increasing analyzer adoption in Eastern European pharmaceutical facilities and growing food testing laboratories implementing automated analytical systems.
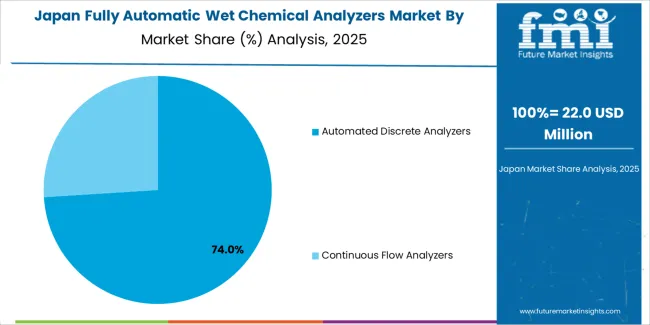
Japanese fully automatic wet chemical analyzers operations reflect the country's exacting quality standards and sophisticated analytical expectations. Major pharmaceutical manufacturers including Takeda, Astellas, and Daiichi Sankyo maintain rigorous equipment qualification processes that often exceed international standards, requiring extensive validation documentation, performance verification testing, and facility audits that can take 18-24 months to complete. This creates high barriers for new analyzer suppliers but ensures consistent analytical quality that supports pharmaceutical product reliability.
The Japanese market demonstrates unique application preferences, with significant demand for precisely controlled measurement protocols and specific detection limit specifications tailored to pharmacopeial requirements. Companies require exact accuracy specifications and traceability parameters that differ from Western applications, driving demand for customized validation protocols and comprehensive technical support.
Regulatory oversight through the Pharmaceuticals and Medical Devices Agency emphasizes comprehensive analytical method validation and equipment qualification requirements that surpass most international standards. The pharmaceutical registration system requires detailed analytical documentation, creating advantages for suppliers with transparent validation processes and comprehensive quality assurance systems.
Supply chain management focuses on relationship-based partnerships rather than purely transactional procurement. Japanese companies typically maintain long-term supplier relationships spanning decades, with annual service contracts emphasizing analytical consistency and technical support over price competition. This stability supports investment in specialized validation protocols tailored to Japanese regulatory requirements and enables collaborative method development programs.
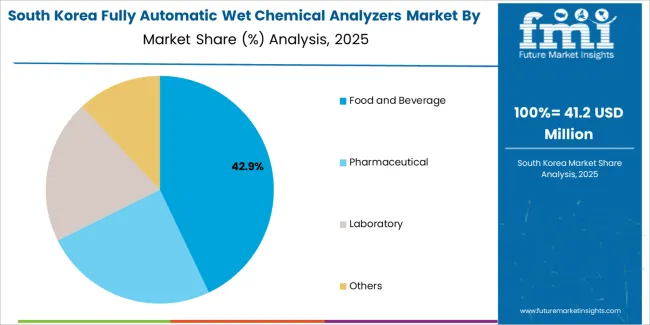
South Korean fully automatic wet chemical analyzers operations reflect the country's advanced pharmaceutical manufacturing sector and research-oriented business model. Major pharmaceutical companies including Samsung Biologics, Celltrion, and SK Biopharmaceuticals drive sophisticated analyzer procurement strategies, establishing direct relationships with global suppliers to secure consistent analytical quality and technical support for their operations targeting both domestic and international markets.
The Korean market demonstrates particular strength in applying automated analytical systems to biopharmaceutical testing applications, with companies integrating advanced analytical protocols into quality control programs designed for biosimilar and innovative drug development. This technical focus creates demand for specific analytical specifications that differ from traditional small molecule applications, requiring suppliers to provide comprehensive method development support and validation assistance.
Regulatory frameworks emphasize analytical data integrity and traceability, with Ministry of Food and Drug Safety standards often exceeding international requirements. This creates barriers for smaller analyzer suppliers but benefits established manufacturers who can demonstrate comprehensive validation capabilities. The regulatory environment particularly favors suppliers with detailed documentation systems and quality assurance protocols.
Supply chain efficiency remains critical given Korea's focus on biopharmaceutical manufacturing and analytical service requirements. Companies increasingly pursue long-term service contracts with analyzer suppliers to ensure reliable analytical performance while managing method validation timelines. Technical support investments enable analytical method optimization and regulatory compliance during pharmaceutical development programs.
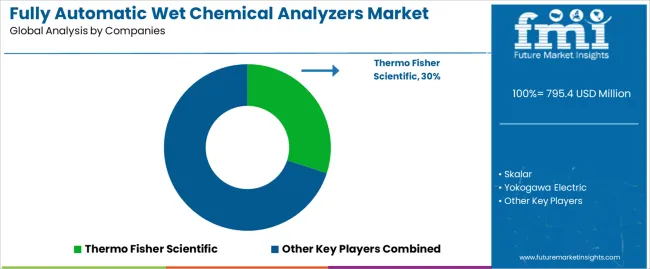
Profit pools are consolidating around integrated manufacturers with comprehensive analytical portfolios and established pharmaceutical industry relationships. Value is migrating from basic analytical equipment to application-specific systems where method validation support, data integrity capabilities, and regulatory compliance documentation command premiums. Several competitive archetypes define market structure: global analytical instrument companies with diversified technology portfolios and comprehensive service networks; specialized wet chemistry analyzer manufacturers focusing on pharmaceutical and food applications with deep application expertise; regional equipment suppliers serving local testing laboratories with cost advantages and responsive support; and contract manufacturers providing private label systems for established brands.
Switching costs stabilize incumbent positions through method validation requirements, regulatory filing dependencies, and operational qualification protocols. Pharmaceutical manufacturers invest 12-18 months validating new analytical systems, creating barriers that protect established suppliers. Performance specifications for detection limits, measurement precision, and analytical throughput establish quality thresholds that limit competitive entry. However, technology advances in sample handling automation and data management systems periodically create opportunities for innovative suppliers.
Market dynamics favor manufacturers with comprehensive validation documentation spanning multiple regulatory jurisdictions. Technical service capabilities that support method development, equipment qualification, and troubleshooting create differentiation in pharmaceutical segments. Supply chain integration from component manufacturing through final system assembly enables quality control and cost management advantages. Geographic proximity to pharmaceutical manufacturing centers reduces response times and enables collaborative method development, particularly valuable in Asia Pacific markets where pharmaceutical production concentrates.
Consolidation continues as analyzer manufacturers seek scale economies to support global service networks and validation documentation development. Vertical integration opportunities attract pharmaceutical companies seeking analytical capabilities, though specialized instrument expertise limits internal development. Digital platforms enable remote diagnostics and predictive maintenance but validation requirements maintain field service importance. Do now: secure pharmaceutical manufacturer partnerships through comprehensive validation support and regulatory documentation; establish service networks in Asia Pacific pharmaceutical manufacturing centers. Option: develop biosimilar-specific analytical methods addressing emerging testing requirements.
| Stakeholder Type | Primary Advantage | Repeatable Plays |
|---|---|---|
| Global instrument companies | Diversified portfolios, service networks | Multi-application systems, validation libraries, global support |
| Specialized analyzer manufacturers | Application expertise, pharmaceutical relationships | Method development, validation support, technical training |
| Regional equipment suppliers | Cost position, local presence | Responsive service, competitive pricing, customization |
| Contract manufacturers | Manufacturing efficiency, private label capabilities | Component integration, cost optimization, quality systems |
| Items | Values |
|---|---|
| Quantitative Units | USD 795.4 million |
| Analyzer Type | Automated Discrete Analyzers, Continuous Flow Analyzers |
| Application | Food and Beverage, Pharmaceutical, Laboratory, Others |
| Regions Covered | North America, Latin America, Europe, Asia Pacific, Middle East & Africa |
| Countries Covered | United States, Germany, China, India, Brazil, United Kingdom, Japan, and other 40+ countries |
| Key Companies Profiled | Thermo Fisher Scientific, Skalar, Yokogawa Electric, AMS Alliance (KPM), Xylem, SEAL Analytical (Porvair), Systea SpA, Ezkem, Astoria-Pacific, Galvanic Applied Sciences |
| Additional Attributes | Dollar sales by analyzer type/application, regional demand, competitive landscape, discrete vs. continuous flow adoption, validation protocol integration, and technical innovation driving analytical precision, automation efficiency, and regulatory compliance |
The global fully automatic wet chemical analyzers market is estimated to be valued at USD 795.4 million in 2025.
The market size for the fully automatic wet chemical analyzers market is projected to reach USD 1,465.3 million by 2035.
The fully automatic wet chemical analyzers market is expected to grow at a 6.3% CAGR between 2025 and 2035.
The key product types in fully automatic wet chemical analyzers market are automated discrete analyzers and continuous flow analyzers.
In terms of application, food and beverage segment to command 43.7% share in the fully automatic wet chemical analyzers market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Fully Enclosed Cartons Market Size and Share Forecast Outlook 2025 to 2035
Fully Enclosed 3D Printing Smart Warehouse Market Size and Share Forecast Outlook 2025 to 2035
Fully Integrated Dishwasher Market Size and Share Forecast Outlook 2025 to 2035
Fully Automated Coagulometer Market
Fully Automatic Hydraulic Lifting Column Market Size and Share Forecast Outlook 2025 to 2035
Fully Automatic Silver Sintering System Market Size and Share Forecast Outlook 2025 to 2035
Fully Automatic High Speed Nail Making Machine Market Size and Share Forecast Outlook 2025 to 2035
Fully Automatic Solid-Liquid Purge Trap Market Size and Share Forecast Outlook 2025 to 2035
Fully Automatic Liquid Metal Printing Machines Market Size and Share Forecast Outlook 2025 to 2035
Automatic Chicken Deboning Machine Market Size and Share Forecast Outlook 2025 to 2035
Automatic Filter Press Solutions Market Size and Share Forecast Outlook 2025 to 2035
Automatic Filter Presses (AFPs) Market Size and Share Forecast Outlook 2025 to 2035
Automatic Riveting Equipment Market Forecast and Outlook 2025 to 2035
Automatic Powder Forming Machine Market Forecast and Outlook 2025 to 2035
Automatic Weigh Price Labeling Machine Market Size and Share Forecast Outlook 2025 to 2035
Automatic Bending Machine Market Size and Share Forecast Outlook 2025 to 2035
Automatic Transmission Market Size and Share Forecast Outlook 2025 to 2035
Automatic Emergency Braking System Market Size and Share Forecast Outlook 2025 to 2035
Automatic Impact Testing Machine Market Size and Share Forecast Outlook 2025 to 2035
Automatic Glue Machine Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA