The global healthcare and laboratory labels market is projected to expand from USD 11.6 billion in 2025 to USD 17.2 billion by 2035, registering a CAGR of 4.1% during the forecast period. In 2024, sales reached USD 11.1 billion.
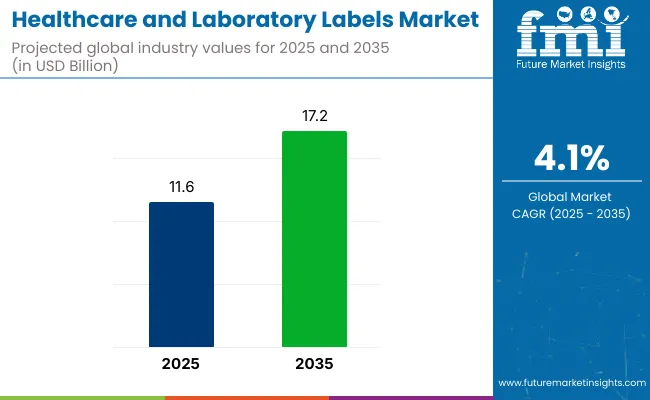
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 11.6 billion |
| Industry Value (2035F) | USD 17.2 billion |
| CAGR (2025 to 2035) | 4.1% |
Growth is being driven by heightened demand for traceability, regulatory compliance, and sample identification in diagnostic laboratories and hospitals. Specialized label technologies offering temperature resistance and sterilization durability have been prioritized across both pharmaceutical and biotechnology sectors, ensuring safe and efficient product tracking.
Labels are essential for a broad spectrum of hospital operations. These include patient wristbands for identification, blood bag labels to ensure correct blood matching, pharmaceutical vial and bottle labeling, and barcode systems for medical device traceability. The ability to withstand harsh environments-such as refrigeration, sterilization, and chemical exposure-is key, especially in labels used for diagnostic samples and long-term storage.
Regulatory mandates from agencies like the USA Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have further emphasized the role of accurate labeling in the supply chain. Labels now must support tamper evidence, serialization, and traceability-especially in light of growing global pharmaceutical trade.
Technological advancement has introduced durable, heat-resistant, and cryo-compatible materials in label production. Moreover, sustainability has emerged as a top concern. Companies are increasingly developing recyclable and linerless label formats, aligning with green healthcare initiatives. Smart labeling technologies such as RFID and QR code integration are also gaining traction, enabling real-time tracking and electronic record synchronization.
In a statement, Kevin Grogan, CEO of RLG Healthcare, underscored the company’s expansion in the sector: “It is an exciting time for RLG Healthcare as we continue to rapidly grow and expand our commitment to the pharmaceutical and healthcare industries” Geographically, North America and Western Europe remain dominant regions due to their advanced healthcare infrastructure and stringent regulations.
However, Asia-Pacific is emerging as a growth hub, supported by rapid hospital network expansion and increasing government investments in digital health and diagnostic capabilities. As healthcare systems grow more complex, the demand for high-performance labeling that ensures patient safety, operational efficiency, and legal compliance will continue to surge, positioning the healthcare and laboratory labels market for sustained global expansion.
The below table presents the expected CAGR for the global healthcare and laboratory labels market over several semi-annual periods spanning from 2025 to 2035. In the first half (H1) of the decade from 2024 to 2034, the business is predicted to surge at a CAGR of 3.8%, followed by a low growth rate of 4.0% in the second half (H2) of the same decade.
| Particular | Value CAGR |
|---|---|
| H1(2024 to 2034) | 3.8% |
| H2(2024 to 2034) | 4.0% |
| H1(2025 to 2035) | 4.0% |
| H2(2025 to 2035) | 4.1% |
Moving into the subsequent period, from H1 2024 to H2 2035, the CAGR is projected to increase to 4.0% in the first half and decrease to 4.1% in the second half. In the first half (H1) the market witnessed an increase of 20 BPS while in the second half (H2), the market witnessed a decrease of 20 BPS.
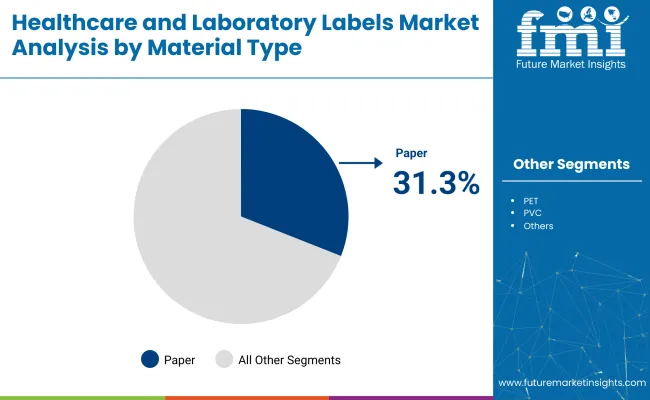
The paper segment is projected to dominate the healthcare and laboratory labels market, accounting for a significant 31.3% market share by 2025. Paper labels are extensively adopted within healthcare settings such as hospitals, diagnostic centers, and laboratories, primarily because of their cost-effectiveness, excellent printability, and ease of compliance with stringent regulatory standards. These labels are predominantly used in patient identification wristbands, specimen containers, drug labeling, and various other critical labeling applications where readability and clarity of information are paramount.
For instance, paper labels with barcode printing are integral in hospital operations for efficient patient tracking and medication management. The versatility of paper labels, allowing both permanent and removable adhesive options, supports their application in both short-term specimen identification and long-term medical record keeping. Prominent healthcare facilities such as Mayo Clinic and Cleveland Clinic utilize paper-based labeling due to their reliability, affordability, and regulatory adherence.
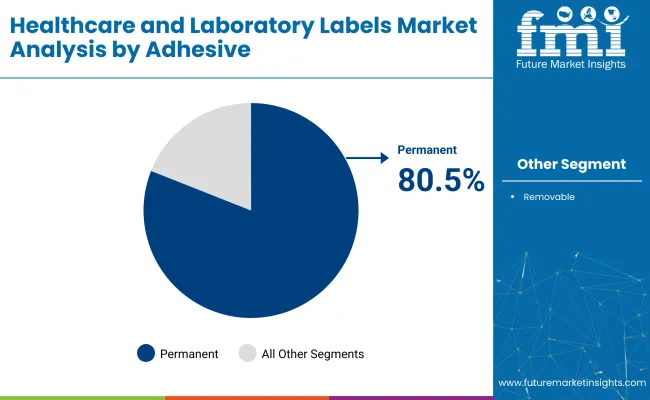
The self-adhesives segment is forecasted to maintain market dominance, capturing a robust 80.5% market share in 2025. Permanent adhesives offer critical advantages in healthcare and laboratory environments, where long-term, secure attachment to containers, medical devices, and specimens is vital. These adhesives are essential for labeling applications like blood bags, medication bottles, and diagnostic samples, as they remain securely affixed under demanding conditions, including refrigeration, sterilization, and chemical exposure.
Laboratories prefer permanent adhesives due to their resistance to harsh handling, thereby minimizing the risk of information loss or contamination. Regulatory compliance also heavily influences the choice of permanent adhesives, especially in applications requiring tamper-evident labeling solutions. Major healthcare institutions, such as Johns Hopkins Hospital and Quest Diagnostics, predominantly use permanent adhesive labels to ensure accurate patient identification and maintain the integrity of critical medical and laboratory data.
Stricter Regulations Drive Demand for Robust, Compliant Healthcare Labeling Solutions
One of the reasons for this is an increased requirement for robust and high-quality labels in health care and laboratories. The healthcare and laboratory industries are unable to survive without such labels, mainly under the pressure of stricter rules and regulations regarding patient safety, tracking of specimens, and identification of medical devices. There are rules and regulations provided by the FDA and ISO stating that labels have to be correct, readable, and resistant to environmental conditions like moisture and chemicals.
For this reason, hospitals, blood banks, diagnostic centers, among others, are always criticized with error cases, adequate identification, and compliance problems for failing to achieve the requirements. Achievement towards labeling requirements: In the durability and tamper evidence of the patient wristband, blood bag, medicine containers, and sample diagnosis, achieving a specialized label solution is significant for the tracing of products along with the patients.
Increased needs to identify the patients and track products have labeled solutions that focus on RFID-enabled labels and the application of label solutions based on barcode technology for filling the demands for regulatory compliances with efficiency in critical operations in health care.
Expansion of Healthcare Facilities Drives Demand for Advanced Labeling Solutions
The growing demand for effective labeling solutions is primarily driven by the development of diagnostic centers, outpatient surgery centers, and veterinary labs. More growth in such healthcare facilities, expansion, and diversification of services result in a higher demand for reliable and accurate systems of labeling to maintain operational efficiency and safety for patients. Diagnostic test tubes, blood bags, surgical instruments, and many other medical products require labels that are not only resistant but also to various forms of exposure, including chemicals, moisture, or extreme temperatures.
Besides, increasing clinical trials further add to the requirement for highly specialized labeling solutions, which can trace samples and tamper evidence on patient data. It is really important to keep track and to identify samples clearly during the process of the clinical trial to be able to guarantee data integrity and to reduce error, thereby being able to avoid patient risk. With expanding health care services, advanced labeling technologies compliant with these needs will still be the biggest factor for making operations run in a smooth fashion and keeping to regulations.
Smaller Healthcare Providers Struggle with Adopting Advanced Labeling Technologies
Independent clinics and ambulatory surgery centers are mostly facing huge challenges in adopting advanced labeling technologies such as a barcode and RFID. Even though these have such obvious benefits for better inventory management, patient tracking, and operational efficiency, innovation occurs at a much slower pace because resources are lacking. High initial expenses for the procurement of advanced printing equipment, special materials, and RFID infrastructure might be too high for smaller facilities with tight budgets.
Moreover, the requirement of training staff and the complexity in integrating these new systems with patient management or inventory systems are some other barriers. Those already in use are likely to use more basic label types, so an upgrade from a more complicated system requires capital outlay as well as some time and support to implement such changes. These reasons make many small healthcare institutions shy away or delay upgrading labeling systems, as more advanced labeling systems have far-reaching benefits to be realized.
Tier 1 company leaders are distinguished by their extensive portfolio and use of advanced production technology. These market leaders are stand out because of their extensive expertise in manufacturing and reconditioning across multiple packaging formats and a broad geographical reach, underpinned by a robust consumer base.
They offer a wide range of series including reconditioning, recycling, and manufacturing utilizing the latest technology and meeting the regulatory standards providing the highest quality. Prominent companies within Tier 1 include CCL Industries Inc., Brady Corporation, Cardinal Health Inc. and Avantor, Inc.
Tier 2 companies are defined by a strong presence overseas and in-depth market knowledge. These market players have good technology and ensure regulatory compliance but may not have advanced technology and wide global reach. Prominent companies in Tier 2 include Sato Holdings Corporation, Schreiner Group, Shamrock Labels, Chicago Tag & Label, Inc., Caresfield, United Ad Label, Zebra Technologies Corporation, CILS International Limited, etc.
Tier 3 includes the majority of small-scale companies operating at the local presence and serving niche markets. These companies are notably oriented towards fulfilling local market demands and are consequently classified within the tier 3 share segment. They are small-scale players and have limited geographical reach. Tier 3, within this context, is recognized as an unorganized market, denoting a sector characterized by a lack of extensive structure and formalization when compared to organized competitors.
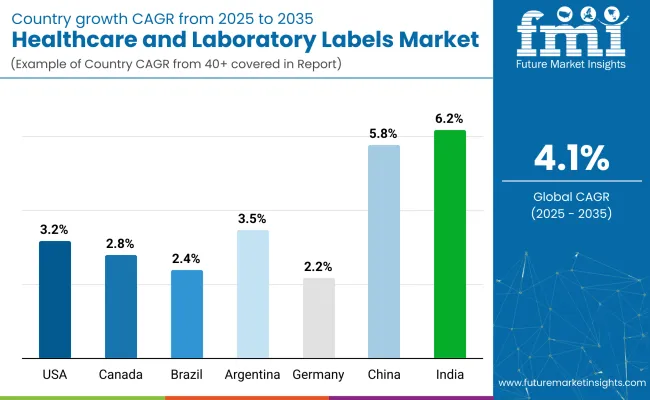
| Countries | Value CAGR (2025 to 2035) |
|---|---|
| USA | 3.2% |
| Canada | 2.8% |
| Brazil | 2.4% |
| Argentina | 3.5% |
| Germany | 2.2% |
| China | 5.8% |
| India | 6.2% |
The section below covers the industry analysis for the healthcare and laboratory labels market for different countries. Market demand analysis on key countries in several regions of the globe, including North America, Asia Pacific, Europe, and others, is provided. USA is anticipated to remain at the forefront in North America, with a CAGR of 3.2% through 2035. In South Asia & Pacific, India is projected to witness a CAGR of 6.2% by 2035.
The rise in the number of hospital admissions and surgeries in the USA, coupled with strict regulations such as the Joint Commission standards, creates a significant force behind the growth of the USA healthcare and laboratory labels market due to the growing need for accuracy in patient identification at hospitals. Advanced labelling solutions, including wristbands, medication labels, and specimen identification tags, which are rugged enough for use under harsh conditions involving moisture, chemicals, or extreme temperatures, are investments that hospitals are willing to undertake.
These also have to integrate with electronic health records (EHR) and barcode scanning systems to ensure efficient tracking of the patient, medication administration, and sample management. As patient safety continues to be a top priority, the acceleration of demand for highly reliable, tamper-evident, and easy-to-scan labels is driving the demand for specialized labeling solutions in healthcare facilities across the United States.
A key driving force for the Germany healthcare and laboratory labels market is the strong emphasis by the country on regulatory compliance and traceability of medical devices. As one of the front-runner countries in the European Union, Germany yields a lot of ground of medical technology ensuring that regulation as stringent as in Medical Device Regulation, MDR, and in-vitro diagnostics, rules all forms of medical devices as well as in laboratory samples issues with information given through labeling accurate and comprehensive on tracing to properly provide patient safe service.
In particular, the demand for tamper-evident labels is strongly rising, especially in surgical instruments, diagnostic tools, and patient samples, which have to be rather resistant to sterilizing processes and aggressive environments. Special labels must therefore be able to withstand moisture, extreme temperatures, and the effects of many chemicals. All these features need the German hospitals and diagnostic laboratories to trace a product all through the supply chain.
This concern about compliance and traceability has increased the need for labeling technologies that are sophisticated by themselves. Such technologies like RFID and bar code solutions can contribute much toward reduction of errors in medical health care and ensure patient safety outcomes.
Key players operating in the healthcare and laboratory labels market are investing in the development of innovative sustainable solutions and also entering into partnerships. Key healthcare and laboratory labels providers have also been acquiring smaller players to grow their presence to further penetrate the healthcare and laboratory labels market across multiple regions.
Key Development
In April 2025, Loftware announced the general availability of two new integrations designed to enhance labeling capabilities within SAP environments. These integrations aim to simplify labeling processes, reduce errors, and ensure regulatory compliance, thereby enabling businesses to achieve greater efficiency and cost savings.
In terms of material type, the industry is divided into polyolefin, PET, PVC, paper, and others (fabric, metallized, etc.).
In terms of printing technology, the industry is segregated into direct thermal, thermal transfer, inkjet printing, and laser printing.
By adhesive, the market is divided into permanent and removable.
The market is classified by end use such as hospitals, laboratories, clinical trials, independent clinics, blood banks, diagnostic centers, veterinary labs, ambulatory surgery centers (ASCs), and outpatient surgery centers.
Key countries of North America, Latin America, East Asia, South Asia & Pacific, Western Europe, Eastern Europe, and the Middle East & Africa have been covered in the report.
The global healthcare and laboratory labels industry is projected to witness CAGR of 4.1% between 2025 and 2035.
The global healthcare and laboratory labels industry stood at USD 4.1 billion in 2024.
The global healthcare and laboratory labels industry is anticipated to reach USD 17.2 billion by 2035 end.
South Asia & Pacific region is set to record the highest CAGR of 5.4% in the assessment period.
The key players operating in the global healthcare and laboratory labels industry include CCL Industries Inc., Brady Corporation, Cardinal Health Inc. and Avantor, Inc.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Healthcare Flooring Market Size and Share Forecast Outlook 2025 to 2035
Healthcare AI Computer Vision Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Business Intelligence Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Master Data Management Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Contact Center Solution Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Semiconductor Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Cold Chain Logistics Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Mobile Computers Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Cloud Infrastructure Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Companion Robots Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Analytical Testing Services Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Analytics Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Contract Research Organization Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
Healthcare Chatbot Market - Growth Trends & Forecast 2025 to 2035
Healthcare Video Conferencing Solutions Market Analysis - Trends & Forecast 2025 to 2035
Healthcare Virtual Assistants Market Analysis by Product, End User and Region Through 2035
Healthcare Natural Language Processing (NLP) Market Insights – Trends & Growth Forecast 2025 to 2035
Healthcare Interoperability Solutions Market Analysis – Trends & Growth 2025 to 2035
Healthcare Digital Experience Platform Market Trends - Growth & Forecast 2025 to 2035
Healthcare API Market Growth – Trends & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA