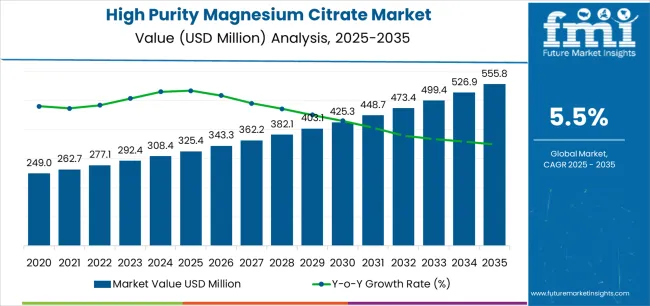
The high purity magnesium citrate market's trajectory from USD 325.4 million in 2025 to USD 555.8 million by 2035 represents robust expansion, demonstrating accelerating adoption of high-grade magnesium compounds and growing investment in nutritional supplement manufacturing across pharmaceutical companies, food processing facilities, and nutraceutical producers worldwide.
The market operates within a dynamic landscape characterized by expanding health consciousness, supplement industry modernization initiatives, and growing demand for pharmaceutical-grade mineral compounds across dietary supplement production, pharmaceutical manufacturing, and functional food development applications. Market dynamics reflect increasing investment in health and wellness infrastructure, accelerating adoption of high-purity mineral supplements, and rising demand for quality-assured magnesium compounds that support diverse therapeutic protocols and nutritional formulations.
Pharmaceutical and nutraceutical procurement patterns demonstrate shifting preferences toward high-purity magnesium citrate systems that combine bioavailability optimization, quality assurance capabilities, and regulatory compliance features. Supplement manufacturers and pharmaceutical companies prioritize ingredient purity, dissolution characteristics, and supply chain reliability when selecting magnesium compounds for critical applications including dietary supplements, pharmaceutical formulations, functional foods, and therapeutic preparations.
The market benefits from expanding health and wellness activities across pharmaceutical, nutraceutical, and functional food sectors, driving demand for sophisticated mineral compounds that enable complex formulation procedures. Growing emphasis on product quality and regulatory compliance creates opportunities for manufacturers offering validated high-purity systems with comprehensive documentation and pharmaceutical-grade quality assurance capabilities.
Technology advancement influences market evolution through integration of advanced purification processes, enhanced testing methodologies, and improved manufacturing systems that optimize product quality and therapeutic outcomes. Manufacturers focus on developing magnesium citrate solutions that accommodate varying purity requirements, particle size specifications, and dissolution parameters while maintaining precise chemical composition throughout extended storage periods.
The high purity magnesium citrate market demonstrates strong growth fundamentals driven by expanding health consciousness, pharmaceutical infrastructure development, and increasing demand for premium mineral supplements across multiple therapeutic disciplines and consumer applications.
The first half of the decade (2025-2030) will witness market growth from USD 325.4 million to approximately USD 403.1 million, adding USD 77.7 million in value, representing 34% of the total forecast period expansion. This phase will be characterized by rapid adoption of pharmaceutical-grade compounds, driven by regulatory compliance programs and increasing demand for high-purity mineral supplements across therapeutic applications.
The latter half (2030-2035) will experience accelerated growth from USD 403.1 million to USD 555.8 million, representing an addition of USD 152.8 million or 66% of the decade's expansion. This period will be defined by mass market penetration of premium magnesium citrate technologies, integration with pharmaceutical manufacturing systems, and seamless connectivity with existing supplement production infrastructure.
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Food grade compounds (supplement, food industry) | 62% | Traditional applications, established facilities |
| Pharmaceutical grade systems | 31% | Premium formulations, regulated applications | |
| Specialty & custom grades | 7% | Premium installations, specialized research | |
| Future (3-5 yrs) | Advanced food grade systems | 58-61% | Enhanced purity, improved bioavailability |
| Pharmaceutical grade compounds | 34-37% | Regulatory compliance, therapeutic applications | |
| Nutrient supplement applications | 28-32% | Dietary supplements, health products | |
| Food & beverage applications | 24-28% | Functional foods, fortification programs | |
| Pharmaceutical applications | 18-22% | Therapeutic formulations, medical nutrition | |
| Industrial applications | 6-10% | Chemical synthesis, specialty compounds | |
| Specialized applications | 4-8% | Custom formulations, research applications |
| Metric | Value |
|---|---|
| Market Value (2025) | USD 325.4 million |
| Market Forecast (2035) | USD 555.9 million |
| Growth Rate | 5.5% CAGR |
| Leading Grade | Food Grade |
| Primary Application | Nutrient Supplement Segment |
The market demonstrates strong fundamentals with food grade systems capturing dominant share through advanced purity control and pharmaceutical application optimization. Nutrient supplement applications drive primary demand, supported by increasing health consciousness and supplement industry expansion initiatives. Geographic distribution remains concentrated in developed markets with established pharmaceutical infrastructure, while emerging economies show accelerating adoption rates driven by health modernization programs and rising wellness investment.
Primary Classification: The market segments by grade into food grade and pharmaceutical grade systems, representing evolution from basic mineral compounds to sophisticated high-purity magnesium citrate technologies for comprehensive therapeutic and nutritional optimization.
Secondary Classification: Application segmentation divides the market into nutrient supplements, food & beverage industry, pharmaceutical industry, and others, reflecting distinct requirements for purity levels, bioavailability characteristics, and formulation specifications.
Tertiary Classification: End-use segmentation covers supplement manufacturers, pharmaceutical companies, food processors, nutraceutical firms, and functional food producers, while distribution channels span direct sales, pharmaceutical ingredient distributors, and specialized chemical suppliers.
Regional Classification: Geographic distribution covers North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, and Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by health infrastructure development programs.
The segmentation structure reveals technology progression from traditional mineral compounds toward sophisticated high-purity magnesium citrate systems with enhanced bioavailability capabilities, while application diversity spans from basic supplementation to specialized pharmaceutical procedures requiring precise chemical composition solutions.
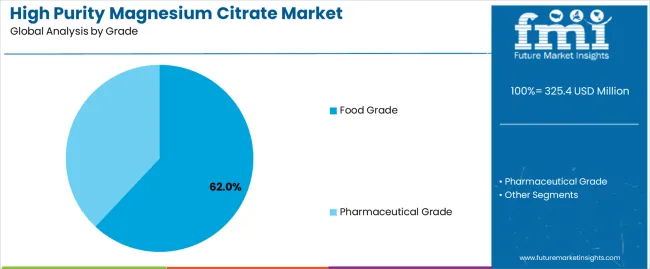
Market Position: Food grade magnesium citrate systems command the leading position in the high purity magnesium citrate market with 62% market share through proven purity control technologies, including efficient manufacturing processes, reliable quality systems, and supplement performance optimization that enable manufacturers to achieve optimal formulation conditions across diverse supplement and food processing environments.
Value Drivers: The segment benefits from supplement industry preference for cost-effective high-purity compounds that provide consistent quality control, regulatory compliance, and formulation flexibility without requiring extensive processing modifications. Advanced food grade processing features enable enhanced bioavailability, precise composition control, and integration with existing manufacturing systems, where product quality and process reliability represent critical operational requirements.
Competitive Advantages: Food grade magnesium citrate systems differentiate through proven manufacturing reliability, regulatory compliance characteristics, and integration with established supplement production systems that enhance formulation effectiveness while maintaining optimal quality standards suitable for diverse consumer applications.
Key market characteristics:
Pharmaceutical grade magnesium citrate systems maintain a 31% market position in the high purity magnesium citrate market due to their specialized quality advantages and therapeutic application benefits. These systems appeal to facilities requiring premium purity solutions with enhanced regulatory compliance profiles for pharmaceutical-scale operations. Market growth is driven by pharmaceutical facility expansion, emphasizing high-quality compound solutions and operational excellence through optimized therapeutic effectiveness designs.
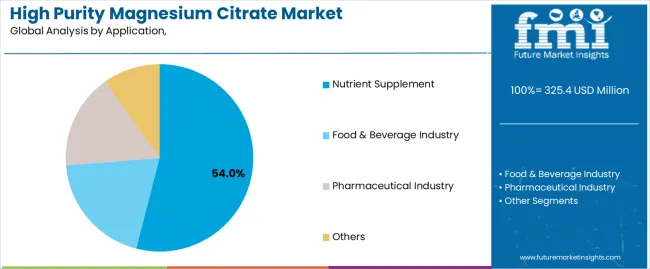
Market Context: Nutrient supplement applications demonstrate strong growth in the high-purity magnesium citrate market, holding a 54% share, due to the widespread adoption of dietary supplement technologies and the increasing focus on health and wellness products, mineral supplementation programs, and therapeutic nutrition applications that maximize bioavailability effectiveness while maintaining consumer safety standards.
Appeal Factors: Supplement manufacturers prioritize ingredient reliability, bioavailability optimization, and integration with existing production infrastructure that enables coordinated manufacturing procedures across multiple product formulations. The segment benefits from substantial health and wellness investment and nutraceutical development programs that emphasize acquisition of high-purity mineral compounds for dietary supplement optimization and therapeutic nutrition applications.
Growth Drivers: Health and wellness programs incorporate magnesium citrate as essential components for supplement formulations, while pharmaceutical development increases demand for mineral compounds that comply with regulatory standards and minimize formulation variability.
Market Challenges: Varying formulation requirements and dosage complexity may limit ingredient standardization across different manufacturing facilities or product scenarios.
Application dynamics include:
Food & beverage applications capture 26% market share through functional food requirements in food processing facilities, beverage manufacturers, and nutritional product applications. These facilities demand high-performance mineral compounds capable of supporting food fortification protocols while providing bioavailability optimization and processing reliability capabilities.
Pharmaceutical applications account for 20% market share, including therapeutic formulations, medical nutrition products, and pharmaceutical manufacturing operations requiring precise mineral compounds for drug formulation and medical nutrition optimization.
Market Context: Supplement Manufacturers dominate the market with 5.8% CAGR, reflecting the primary demand source for high purity magnesium citrate technology in dietary supplement production and formulation optimization.
Business Model Advantages: Supplement Manufacturers provide direct market demand for standardized mineral compounds, driving volume production and cost optimization while maintaining quality control and performance consistency requirements.
Operational Benefits: Supplement Manufacturer applications include formulation optimization, production efficiency, and quality assurance that drive consistent demand for mineral compounds while providing access to latest magnesium citrate technologies.
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Health consciousness expansion & supplement industry growth (wellness trends, preventive healthcare) | ★★★★★ | Growing wellness market requires high-purity mineral compounds with enhanced bioavailability capabilities and therapeutic properties proven effective across supplement applications. |
| Driver | Pharmaceutical advancement & nutraceutical development (drug formulation, therapeutic nutrition) | ★★★★★ | Transforms supplement requirements from "basic minerals" to "pharmaceutical-grade compounds"; manufacturers that offer high-purity systems and bioavailability features gain competitive advantage. |
| Driver | Regulatory compliance & quality standardization (FDA requirements, pharmaceutical standards) | ★★★★☆ | Modern pharmaceutical facilities need validated, compliant mineral compounds; demand for certified and documented supplement ingredients expanding addressable market. |
| Restraint | High purification cost & price sensitivity (especially for basic supplement manufacturers) | ★★★★☆ | Smaller supplement manufacturers defer high-purity compound upgrades; increases price sensitivity and slows premium ingredient adoption in cost-conscious markets. |
| Restraint | Alternative magnesium compound competition (oxide, sulfate, other citrate sources) | ★★★☆☆ | Traditional magnesium alternatives offer established supply chains and lower costs, potentially limiting high-purity citrate adoption in conventional applications. |
| Trend | Bioavailability enhancement & formulation optimization (absorption properties, therapeutic effectiveness) | ★★★★★ | Advanced bioavailability properties, dissolution optimization, and therapeutic analytics transform operations; ingredient enhancement and performance improvement become core value propositions. |
| Trend | Manufacturing integration & quality monitoring (process automation, quality systems) | ★★★★☆ | Smart manufacturing systems for specific applications and formulations; specialized monitoring and targeted optimization capabilities drive competition toward integrated solutions. |
The high purity magnesium citrate market demonstrates varied regional dynamics with Growth Leaders including China (7.4% growth rate) and India (6.9% growth rate) driving expansion through pharmaceutical infrastructure development and supplement industry modernization initiatives. Steady Performers encompass Germany (6.3% growth rate), Brazil (5.8% growth rate), and developed regions, benefiting from established pharmaceutical facilities and nutraceutical sector growth. Mature Markets feature United States (5.2% growth rate), United Kingdom (4.7% growth rate), and Japan (4.1% growth rate), where health advancement and supplement optimization requirements support consistent growth patterns.
Regional synthesis reveals East Asian markets leading adoption through pharmaceutical expansion and nutraceutical development, while North American countries maintain steady expansion supported by supplement industry advancement and health infrastructure investment. European markets show strong growth driven by pharmaceutical applications and regulatory compliance trends.
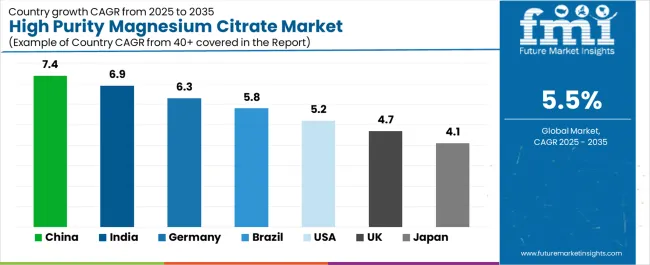
| Region/Country | 2025-2035 Growth | How to win | What to watch out |
|---|---|---|---|
| China | 7.4% | Focus on cost-effective pharmaceutical solutions | Regulatory changes; local competition |
| India | 6.9% | Lead with bioavailability systems | Import restrictions; infrastructure barriers |
| Germany | 6.3% | Provide premium pharmaceutical systems | Over-regulation; lengthy approvals |
| Brazil | 5.8% | Offer value-oriented solutions | Currency fluctuations; import duties |
| United States | 5.2% | Push quality integration | Compliance costs; scaling challenges |
| United Kingdom | 4.7% | Focus on pharmaceutical applications | Economic impacts; funding constraints |
| Japan | 4.1% | Emphasize precision manufacturing | Traditional preferences; adoption rates |
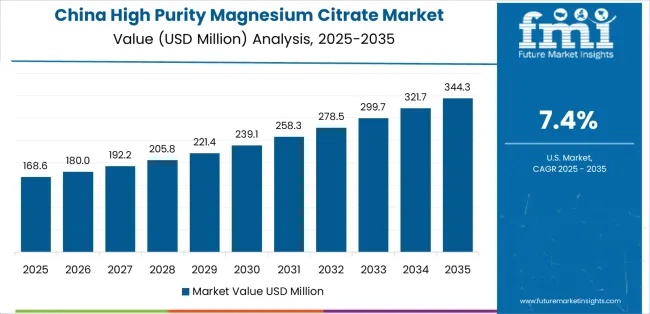
China establishes fastest market growth through aggressive pharmaceutical infrastructure development programs and comprehensive supplement industry expansion, integrating high-purity magnesium citrate systems as standard components in pharmaceutical manufacturing and supplement production facilities. The country's 7.4% growth rate reflects government initiatives promoting health infrastructure and domestic nutraceutical capabilities that mandate use of pharmaceutical-grade mineral compounds in supplement manufacturing and therapeutic product facilities. Growth concentrates in major pharmaceutical hubs, including Beijing, Shanghai, and Guangzhou, where manufacturing development showcases integrated production systems that appeal to supplement manufacturers seeking bioavailability optimization capabilities and pharmaceutical applications.
Chinese manufacturers are developing cost-effective mineral compound solutions that combine domestic production advantages with advanced purification features, including enhanced bioavailability control and improved therapeutic capabilities. Distribution channels through pharmaceutical ingredient suppliers and supplement facility integrators expand market access, while government support for health development supports adoption across diverse pharmaceutical and nutraceutical segments.
Strategic Market Indicators:
In Mumbai, Delhi, and Bangalore, pharmaceutical facilities and supplement manufacturers are implementing high purity magnesium citrate systems as standard ingredients for formulation optimization and therapeutic effectiveness applications, driven by increasing government health investment and pharmaceutical development programs that emphasize importance of quality mineral compound capabilities.
The market holds a 6.9% growth rate, supported by government health initiatives and pharmaceutical modernization programs that promote high-purity mineral compounds for pharmaceutical and supplement facilities. Indian manufacturers are adopting mineral compound systems that provide consistent bioavailability optimization and therapeutic features, particularly appealing in urban regions where health optimization and supplement excellence represent critical pharmaceutical requirements.
Market expansion benefits from growing pharmaceutical capabilities and international technology partnerships that enable domestic production of high-purity mineral compounds for supplement and pharmaceutical applications. Technology adoption follows patterns established in pharmaceutical ingredients, where quality and bioavailability drive procurement decisions and operational deployment.
Market Intelligence Brief:
Advanced pharmaceutical market in Germany demonstrates sophisticated magnesium citrate deployment with documented bioavailability effectiveness in nutraceutical applications and pharmaceutical facilities through integration with existing manufacturing systems and pharmaceutical infrastructure. The country leverages engineering expertise in pharmaceutical technologies and mineral compound integration to maintain a 6.3% growth rate. Manufacturing centers, including Bavaria, Baden-Württemberg, and North Rhine-Westphalia, showcase premium installations where mineral compound systems integrate with comprehensive pharmaceutical platforms and facility management systems to optimize manufacturing efficiency and therapeutic effectiveness.
German manufacturers prioritize system quality and EU compliance in pharmaceutical development, creating demand for premium systems with advanced features, including facility integration and quality management systems. The market benefits from established pharmaceutical infrastructure and willingness to invest in high-purity mineral compounds that provide long-term operational benefits and compliance with international pharmaceutical standards.
Market Intelligence Brief:
Brazil's market expansion benefits from diverse health demand, including pharmaceutical modernization in São Paulo and Rio de Janeiro, supplement facility upgrades, and government health programs that increasingly incorporate high-purity mineral compounds for therapeutic applications. The country maintains a 5.8% growth rate, driven by rising health activity and increasing recognition of bioavailability optimization benefits, including accurate therapeutic control and enhanced supplement effectiveness.
Market dynamics focus on cost-effective mineral compound solutions that balance bioavailability performance with affordability considerations important to Brazilian pharmaceutical manufacturers. Growing health industrialization creates continued demand for modern mineral compounds in new pharmaceutical infrastructure and facility modernization projects.
Strategic Market Considerations:
United States establishes market leadership through comprehensive health programs and advanced pharmaceutical infrastructure development, integrating magnesium citrate systems across supplement and pharmaceutical applications. The country's 5.2% growth rate reflects established pharmaceutical relationships and mature ingredient technology adoption that supports widespread use of high-purity mineral compounds in supplement and pharmaceutical facilities. Growth concentrates in major pharmaceutical centers, including California, Massachusetts, and North Carolina, where pharmaceutical technology showcases mature deployment that appeals to supplement manufacturers seeking proven bioavailability capabilities and therapeutic optimization applications.
American pharmaceutical providers leverage established distribution networks and comprehensive technical support capabilities, including system design programs and training support that create customer relationships and operational advantages. The market benefits from mature regulatory standards and pharmaceutical requirements that mandate mineral compound use while supporting technology advancement and therapeutic optimization.
Market Intelligence Brief:
United Kingdom's pharmaceutical market demonstrates integrated magnesium citrate deployment with documented bioavailability effectiveness in supplement applications and pharmaceutical facilities through integration with existing manufacturing systems and pharmaceutical infrastructure. The country maintains a 4.7% growth rate, supported by pharmaceutical efficiency programs and therapeutic effectiveness requirements that promote high-purity mineral compounds for pharmaceutical applications. Manufacturing facilities across England, Scotland, and Wales showcase systematic installations where mineral compound systems integrate with comprehensive pharmaceutical platforms to optimize bioavailability and therapeutic outcomes.
UK pharmaceutical providers prioritize ingredient reliability and manufacturing compatibility in mineral compound procurement, creating demand for validated systems with proven bioavailability features, including performance monitoring integration and quality management systems. The market benefits from established pharmaceutical infrastructure and quality requirements that support pharmaceutical ingredient adoption and therapeutic effectiveness.
Market Intelligence Brief:
Japan's market growth benefits from precision pharmaceutical demand, including advanced supplement facilities in Tokyo and Osaka, quality integration, and bioavailability enhancement programs that increasingly incorporate mineral compound solutions for therapeutic applications. The country maintains a 4.1% growth rate, driven by pharmaceutical technology advancement and increasing recognition of high-purity mineral compound benefits, including accurate bioavailability control and enhanced therapeutic outcomes.
Market dynamics focus on high-precision mineral compound solutions that meet Japanese quality standards and bioavailability effectiveness requirements important to pharmaceutical manufacturers. Advanced pharmaceutical technology adoption creates continued demand for sophisticated mineral compound systems in pharmaceutical facility infrastructure and therapeutic modernization projects.
Strategic Market Considerations:
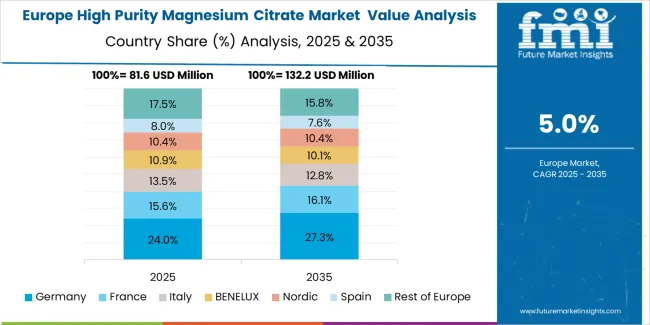
The European high purity magnesium citrate market is projected to grow from USD 62.1 million in 2025 to USD 89.4 million by 2035, registering a CAGR of 3.7% over the forecast period. Germany is expected to maintain its leadership position with a 38.6% market share in 2025, supported by its advanced pharmaceutical infrastructure and major nutraceutical centers.
United Kingdom follows with a 24.8% share in 2025, driven by comprehensive pharmaceutical programs and supplement development initiatives. France holds a 16.4% share through specialized pharmaceutical applications and regulatory compliance requirements. Italy commands a 11.2% share, while Spain accounts for 6.7% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 2.3% to 2.6% by 2035, attributed to increasing pharmaceutical adoption in Nordic countries and emerging supplement facilities implementing manufacturing modernization programs.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global brands | Distribution reach, broad product catalogs, brand recognition | Wide availability, proven bioavailability, multi-region support | Product refresh cycles; customer dependency on brand validation |
| Technology innovators | Purification R&D; advanced compound technologies; enhanced bioavailability properties | Latest technologies first; attractive ROI on therapeutic effectiveness | Service density outside core regions; scaling complexity |
| Regional specialists | Local compliance, fast delivery, nearby customer support | "Close to customer" support; pragmatic pricing; local regulations | Technology gaps; talent retention in customer service |
| Full-service providers | Complete pharmaceutical programs, system integration, quality monitoring | Lowest operational risk; comprehensive support | Service costs if overpromised; technology obsolescence |
| Niche specialists | Specialized applications, custom compounds, pharmaceutical services | Win premium applications; flexible configurations | Scalability limitations; narrow market focus |
| Item | Value |
|---|---|
| Quantitative Units | USD 325.4 million |
| Grade | Food Grade, Pharmaceutical Grade |
| Application | Nutrient Supplement, Food & Beverage Industry, Pharmaceutical Industry, Others |
| End Use | Supplement Manufacturers, Pharmaceutical Companies, Food Processors, Nutraceutical Firms, Functional Food Producers |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, Middle East & Africa |
| Countries Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, Canada, France, Australia, and 25+ additional countries |
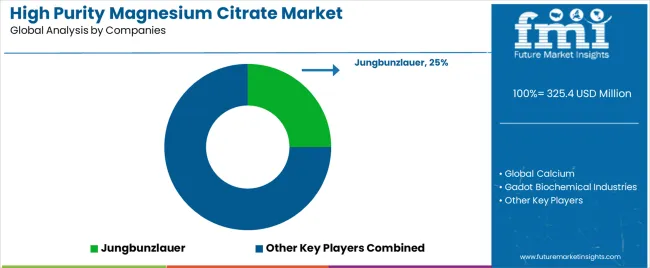
| Key Companies Profiled | Jungbunzlauer, Global Calcium, Gadot Biochemical Industries, Sucroal, Dr. Paul Lohmann, Penglai Marine, Dongtai Food Ingredients, Nantong Feiyu Food Technology |
| Additional Attributes | Dollar sales by grade and application categories, regional adoption trends across East Asia, North America, and Western Europe, competitive landscape with pharmaceutical ingredient manufacturers and system integrators, supplement manufacturer preferences for bioavailability effectiveness and purity control, integration with manufacturing platforms and quality management systems, innovations in mineral compound technology and therapeutic enhancement, and development of advanced magnesium citrate solutions with enhanced bioavailability and therapeutic optimization capabilities. |
The global high purity magnesium citrate market is estimated to be valued at USD 325.4 million in 2025.
The market size for the high purity magnesium citrate market is projected to reach USD 555.9 million by 2035.
The high purity magnesium citrate market is expected to grow at a 5.5% CAGR between 2025 and 2035.
The key product types in high purity magnesium citrate market are food grade and pharmaceutical grade.
In terms of application,, nutrient supplement segment to command 54.0% share in the high purity magnesium citrate market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
High-performance Dual-core Processor Market Size and Share Forecast Outlook 2025 to 2035
High Performance Magnet Market Size and Share Forecast Outlook 2025 to 2035
High-frequency RF Evaluation Board Market Size and Share Forecast Outlook 2025 to 2035
High Viscosity Mixer Market Size and Share Forecast Outlook 2025 to 2035
High Voltage Ionising Air Gun Market Size and Share Forecast Outlook 2025 to 2035
High Voltage Equipment Market Forecast and Outlook 2025 to 2035
High Clear Film Market Size and Share Forecast Outlook 2025 to 2035
High Performance Random Packing Market Forecast Outlook 2025 to 2035
High Precision Microfluidic Pump Market Size and Share Forecast Outlook 2025 to 2035
High Performance Composites Market Forecast Outlook 2025 to 2035
High Performance Medical Plastic Market Forecast Outlook 2025 to 2035
High Temperature Heat Pump Dryers Market Size and Share Forecast Outlook 2025 to 2035
High Temperature Fiberglass Filter Media Market Size and Share Forecast Outlook 2025 to 2035
High Mast Lighting Market Forecast and Outlook 2025 to 2035
High-Protein Pudding Market Forecast and Outlook 2025 to 2035
High Voltage Ceramic Zinc Oxide Surge Arrester Market Size and Share Forecast Outlook 2025 to 2035
High-Power Microwave Source Market Size and Share Forecast Outlook 2025 to 2035
High Performance Epoxy Coating Market Size and Share Forecast Outlook 2025 to 2035
High Molecular Ammonium Polyphosphate Market Size and Share Forecast Outlook 2025 to 2035
High Performance Fluoropolymer Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA