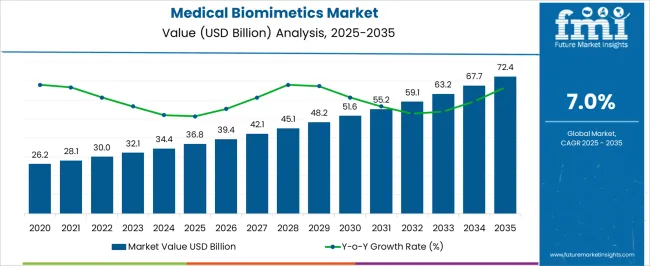
The Medical Biomimetics Market is estimated to be valued at USD 36.8 billion in 2025 and is projected to reach USD 72.4 billion by 2035, registering a compound annual growth rate (CAGR) of 7.0% over the forecast period.
| Metric | Value |
|---|---|
| Medical Biomimetics Market Estimated Value in (2025 E) | USD 36.8 billion |
| Medical Biomimetics Market Forecast Value in (2035 F) | USD 72.4 billion |
| Forecast CAGR (2025 to 2035) | 7.0% |
The medical biomimetics market is gaining momentum due to growing clinical demand for regenerative and biocompatible solutions that mimic the structure and function of natural biological systems. This innovation driven domain is transforming therapeutic approaches in areas such as tissue engineering, cardiovascular interventions, and targeted healing by enabling improved integration with human physiology.
Rising investments in advanced biomaterials and interdisciplinary collaborations between biomedical engineering and clinical sciences are accelerating product development. Additionally, aging populations, increasing prevalence of chronic disorders, and demand for minimally invasive treatments are fueling adoption across global healthcare settings.
Technological advancements in nanostructured surfaces and smart materials are expanding the application scope, while supportive regulatory frameworks and academic research programs are further validating clinical efficacy. The outlook remains strong as biomimetic technologies continue to bridge the gap between artificial implants and natural biological function, shaping the future of personalized medicine and next generation therapeutic strategies.
The market is segmented by Type and Application and region. By Type, the market is divided into Cardiovascular, Orthopaedic, Ophthalmology, Dental, and Others. In terms of Application, the market is classified into Wound Healing, Tissue Engineering, Drug Delivery, and Other Applications. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
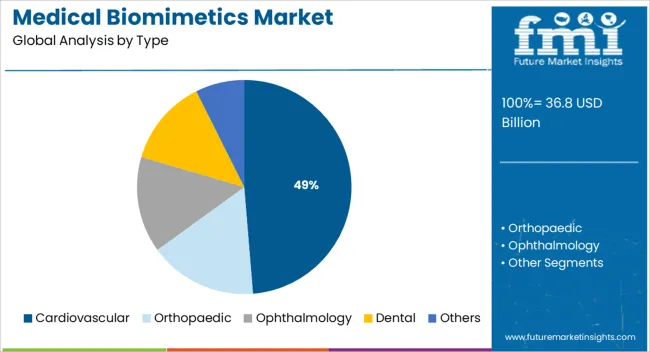
The cardiovascular segment is expected to account for 48.70% of total market revenue by 2025 within the type category, positioning it as the leading segment. This growth is attributed to the rising incidence of cardiovascular diseases and the critical need for advanced implants and prosthetics that replicate natural blood flow dynamics and tissue integration.
Biomimetic cardiovascular devices, such as stents, heart valves, and vascular grafts, are engineered to reduce thrombogenicity and enhance long term biocompatibility. The increasing success of such innovations in clinical trials and real world applications has driven adoption in interventional cardiology and surgical repair.
Furthermore, advancements in surface coatings and material flexibility have enabled improved patient outcomes and device longevity. As hospitals and research institutions prioritize innovation in cardiovascular care, this segment continues to lead the market with strong clinical relevance and commercial viability.
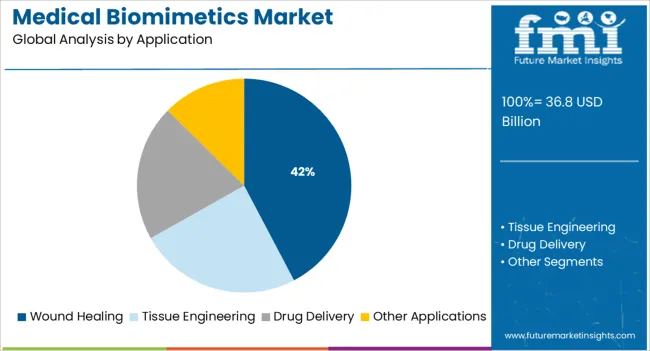
The wound healing segment is projected to contribute 42.30% of total market revenue by 2025 under the application category, making it a prominent area of growth. This expansion is driven by the growing burden of chronic wounds, diabetic ulcers, and post surgical healing challenges that require enhanced tissue regeneration and infection control.
Biomimetic solutions are being developed to replicate extracellular matrix functions, stimulate cellular activity, and promote faster tissue repair. These technologies are increasingly used in advanced wound dressings, skin grafts, and bioengineered scaffolds that support the natural healing environment.
The demand for personalized and cost effective wound care across outpatient clinics, hospitals, and home care settings has further strengthened the adoption of biomimetic applications. The convergence of material science, cell biology, and clinical needs is reinforcing the role of this segment in transforming wound care practices worldwide.
According to market research and competitive intelligence provider Future Market Insights- the market for medical biomimetics reflected a value of 7% during the historical period, 2020 to 2025.
The medical biomimetics market was affected by COVID-19 owing to delays and cancellations of orthopedic, dental, cardiovascular, and other medical procedures. However, the development of vaccines, medicines, and conventional drugs, in addition to the use of innovative drug delivery technologies has increased impetus in the fight against the COVID-19 pandemic.
Advanced delivery technologies, such as the use of nanocapsules/ nanospheres, nanocrystals, liposomes, nano lipid carriers/solid lipid nanoparticles, nanosponges, and dendrimers based on biomimetics can be utilized for targeted delivery of therapeutic compounds to infected individuals for treating COVID-19. Numerous research activities have been carried out for the use of biomimicry during the pandemic.
For instance, in August 2024, researchers from the Academy of Medical Engineering and Translational Medicine, School of Life Sciences, and Tianjin University designed the inhalable nano vaccine with biomimetics COVID-19 structure to activate the mucosal immunity of the respiratory tract against coronavirus.
Through replicating the route of infection and structure, this inhalable nano vaccine approach might encourage a new approach to respiratory virus precaution. Thus, the market for medical biomimetics is expected to register a CAGR of 5% in the forecast period 2025 to 2035.
Surgical devices and instruments development propelling the growth of the medical biomimetics market
Medical biomimetics is the fastest-expanding field of bioengineering. It is the impersonation of features found in animals and plants to provide both treatment and protection from bacteria and illness as well as management of the disease.
Medical biomimetics is considered a technology that emulates or uses nature for the advancement of human lives. Medical biomimetics is gaining more adoption in surgical devices and instrument development.
There has been increasing in the use of biomimetics in tools and devices for the artificial creation of organs mainly due to the susceptibility of old age people to organ dysfunction and failure. Increasing awareness and adoption of biomimetics has had a positive impact on organ transplantation and will continue to push the market growth in forthcoming years.
Efforts to encourage organ donation must continue in all countries, but it is also necessary to develop alternative solutions, such as bioengineering. Various companies are involved in the development of biomimetics-based products.
For instance, CorNeat Vision developed the CorNeat KPro implant, a patented synthetic cornea, which offers a long-lasting medical solution for corneal injury, and blindness. The product is following a CE marking and 510K clearance path, which is expected to be approved for marketing in late 2025. In addition, the company in October 2024 also received USD 2.44 Billion grant from the European Innovation Council to support the clinical activities as well as automation and manufacturing processes which will enable the company to reach the market with this cure.
Advancements in Drug Delivery Systems boosting the adoption of Medical Biomimetics
Moreover, the innovations in nanotechnology, leading to the advancement in drug delivery systems will positively impact the medical biomimetics growth in the forecast period. Based on their biocompatibility physical, and chemical properties, nanomaterials are attractive for use in biomedical applications and exhibit great possibility in biomimetics medicine.
Currently, with the advancement of biomimetic functionalized nanotechnology, nanomaterials have been functionalized and modified by cell membrane-derived elements to form biomimetic nanomaterials with augmented biocompatibility, stability, targeting, and specificity.
The biomimetic functionalized variation of nanomaterials can establish selectivity for tissue disease or biomarkers, implying that these biomimetic nanomaterials can be used for the diagnosis of disease. Thus, the use of biomimetic nanomaterials for the non-invasive diagnosis of diseases has stimulated great interest.
Additionally, rising Research and Development activities coupled with the growing application potential of medical biomimetics in the diagnostic imaging market space including ultrasound, ECGs, and CT scans are expected to drive the market growth.
For instance, Imperial College London is currently developing a biomimetic flexible steerable probe. The objective of this research is to access deep brain areas with the least damage to precisely place the minimally invasive instrumentation (electrodes, catheters for deep stimulation of the brain), to perform the diagnosis and clinical analysis (sampling, biopsy), micro neurosurgery, and localized drug delivery.
Regulatory Frameworks restraining Medical Biomimetics Market Growth
A stringent regulatory framework hinders the medical biomimetics industry's growth. Regarding the ever-increasing and widespread applications of biomimetic biomaterials in numerous medical fields, their precise assessment is of great significance.
Although each biomaterial undergoes thorough premarket evaluation, the regulatory organizations receive a significant number of adverse events and complications reports each year. Moreover, biomaterials are integrated into biological systems, hence, they must fulfill all scientific requirements such as high biocompatibility, sufficiently long shelf-life, and accommodating biological interactions.
Adoption of Novel Technology shaping landscape for Medical Biomimetics
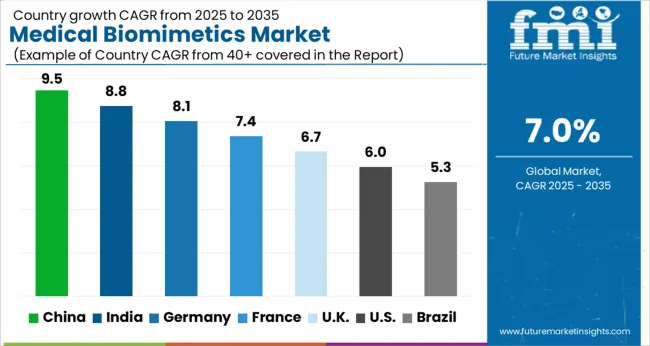
The large share of North America is mainly attributed to the high research and development activities in the region, high adoption of novel technology, high disposable income, and presence of key market players in the region.
North America is expected to be a major market for medical biomimetics due to trends such as an increase in the rate of adoption of novel technologies, growth in the geriatric population, an increase in research and development activities related to medical biomimetics, and a strong presence of key players. Thus, North America is expected to dominate the regional medical biomimetics market with a share of 39% in 2025.
Booming Biotechnology Sector contributing to Medical Biomimetics Growth
The market in the Asia Pacific is expected to expand at a high growth rate during the forecast period due to factors including an increase in government initiatives in the healthcare sector, an increase in the medical tourism sector, and a booming biotechnology industry.
Asia Pacific region is projected to exhibit a high growth rate of the medical biomimetics market during the forecast period owing to increasing investment in medical and healthcare infrastructure, a huge patient population, growing awareness and adoption of new technology, and government initiatives to boost the growth of the biotechnology sector.
Thus, Asia Pacific is expected to possess a 36% market share of the medical biomimetics market in 2025.
Government Funding creating Lucrative Opportunities for use of Medical Biomimetics for Cardiovascular Diseases
CVD is the number one cause of death globally, affecting 17.9 Billion deaths every year. Worldwide, more than 2.1 Billion patients receive CVD implants each year to prevent death. Moreover, the launch of technologically advanced products coupled with government funding offers lucrative opportunities for the segment during the forecast period.
LifeMatrix has developed a distinctive bioengineering technology to grow human replacement tissues as next-generation implants for treating CVDs. The patented LifeMatrix tissues can be manufactured as blood vessels, heart valves, and other cardiovascular structures. The biomimetic device helps to prevent repetitive major surgeries and the risks associated with them throughout the patient’s life.
In addition, the technology which has been created by a team of cardiovascular and cardiac experts in April 2024 also received USD 149,707 in funding from Venture Kick to support regulatory submissions and increase the clinical launch strategy. Thus, by product, the cardiovascular segment is expected to possess a 43% market share for the medical biomimetics market in 2025.
Technological Advancements in Orthopaedic Products spurring the growth of Medical Biomimetics
The orthopedic segment is expected to grow at the fastest CAGR of the medical biomimetics market during the forecast period. Technological advancements coupled with the launch of new products drive the growth of the segment. For instance, in March 2024, Sparta Biomedical Inc. received Breakthrough Device Designation from the USFDA for SBM-01 Biomimetic Implant.
The implant is proposed to replace the damaged knee cartilage in patients suffering from single or multiple osteochondral or chondral defects in the knee. The company, in December 2024, also received USD 5 Billion to advance its preclinical activities for investigational device exemption submission.
Demographic trends contributing to the growth of the application of Medical Biomimetics in Wound Healing
The demand for wound healing and wound care products is growing, owing to the escalating number of surgical cases and the increasing prevalence of chronic diseases globally. Moreover, the segment will continue to grow due to specific demographic trends, with an aging population, rising incidence of diabetes and obesity, and related higher sensitivity towards non-healing chronic wounds. In the USA, in 2024, the prevalence of chronic wounds was about 2% of the overall USA. population or about 6.9 Billion people were suffering from chronic wounds.
Furthermore, the expanding number of patients needing improved treatment represents a considerable burden of cost on the healthcare system. The total cost of treating chronic wounds is increasing sharply, and the recent annual projected cost in the USA exceeds USD 36.8 billion. Thus, the wound healing segment is expected to dominate the medical biomimetics market and expected to account for 35% market share in 2025.
While numerous approaches for wound healing are offered, they are only somewhat effective. Thus, there is a demand for more efficient therapies for healing wounds. The advancement of instructive materials and biomimetics is evolving as a favorable procedure for redirecting fibrotic wound healing into a regenerative method. Biomimetics materials are currently advancing on the shortcomings of current treatments for wound care as they are being created to accelerate these spatiotemporal cues, thus encouraging regeneration within the host tissues.
Demand for Advanced Drug Administration Systems increasing use for Drug Delivery
The drug delivery segment is anticipated to witness a considerable growth rate in the medical biomimetics market during the forecast period. This can be attributed to the patient shifts towards the adoption of advanced drug administration systems, and increasing research and development. So far, albumin-based delivery is perhaps the most successful approach to biomimetics therapeutics, and there are numerous marketed drugs involving Abraxane, liraglutide, semaglutide, and, albiglutide.
In addition, cell-based biomimetic delivery is another interesting case for biomimetic therapeutics. For instance, since the 1970s RBCs as living cell carriers have been examined for decades. Moreover, the adoption of targeted drug delivery systems further promotes the growth of biomimetics in drug delivery applications as biomimetics approaches show promising results in promoting drug efficiency in targeted drug delivery.
Key players in the medical biomimetics market are Biome Renewables, Bimitech, denovoMATRIX, BEOnChip, and Bioxegy
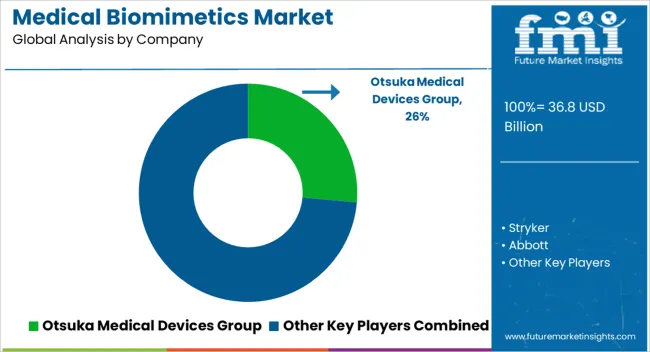
Key players in medical biomimetics are implementing various strategies including partnerships through mergers and acquisitions, geographical expansion, and strategic collaborations to expand their market presence.
Some of the key players in the global medical biomimetics market include Otsuka Medical Devices Group, Stryker, Abbott, AVINENT Science and Technology, SynTouch Inc, Osteopore International Pte Ltd, Vandstrom, Inc., Biomimetics Technologies Inc, Swedish Biomimetics 3000 ApS, Keystone Dental Group, LifeMatrix, Curasan, Inc, CorNeat Vision, NanoHive Medical LLC, Zimmer Biomet
| Report Attribute | Details |
|---|---|
| Market Value in 2025 | USD 36.8 billion |
| Market Value in 2035 | USD 72.4 billion |
| Growth Rate | CAGR of 7% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2025 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in USD Billion and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends, and Pricing Analysis |
| Segments Covered | Type, Application, Region |
| Regions Covered | North America; Latin America; Europe; South Asia; East Asia; Oceania; Middle East and Africa |
| Key Countries Profiled | USA, Canada, Brazil, Mexico, Germany, United Kingdom, France, Spain, Italy, India, Malaysia, Singapore, Thailand, China, Japan, South Korea, Australia, New Zealand, GCC Countries, South Africa |
| Key Companies Profiled | Otsuka Medical Devices Group; Stryker; Abbott; AVINENT Science and Technology; SynTouch Inc.; Osteopore International Pte Ltd; Vandstrom, Inc.; Biomimetics Technologies Inc; Swedish Biomimetics 3000 ApS; Keystone Dental Group; LifeMatrix; Curasan, Inc.; CorNeat Vision; NanoHive Medical LLC; Zimmer Biomet |
| Customization | Available Upon Request |
The global medical biomimetics market is estimated to be valued at USD 36.8 billion in 2025.
The market size for the medical biomimetics market is projected to reach USD 72.4 billion by 2035.
The medical biomimetics market is expected to grow at a 7.0% CAGR between 2025 and 2035.
The key product types in medical biomimetics market are cardiovascular, orthopaedic, ophthalmology, dental and others.
In terms of application, wound healing segment to command 42.3% share in the medical biomimetics market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Medical Eye Shield Film Market Size and Share Forecast Outlook 2025 to 2035
Medical Far Infrared Therapy Device Market Size and Share Forecast Outlook 2025 to 2035
Medical Latex Protective Suit Market Size and Share Forecast Outlook 2025 to 2035
Medical Activated Carbon Dressing Market Size and Share Forecast Outlook 2025 to 2035
Medical Coated Roll Stock Market Size and Share Forecast Outlook 2025 to 2035
Medical Billing Outsourcing Market Size and Share Forecast Outlook 2025 to 2035
Medical Pressure Mapping System Market Size and Share Forecast Outlook 2025 to 2035
Medical Chairs Market Size and Share Forecast Outlook 2025 to 2035
Medical Exoskeleton Market Forecast Outlook 2025 to 2035
Medical Display Market Forecast and Outlook 2025 to 2035
Medical Spa Market Size and Share Forecast Outlook 2025 to 2035
Medical Face Shield Market Forecast and Outlook 2025 to 2035
Medical Robot Market Size and Share Forecast Outlook 2025 to 2035
Medical Nutrition Market Forecast and Outlook 2025 to 2035
Medical Wax Market Size and Share Forecast Outlook 2025 to 2035
Medical Specialty Bag Market Size and Share Forecast Outlook 2025 to 2035
Medical Plastics Market Size and Share Forecast Outlook 2025 to 2035
Medical Device Tester Market Size and Share Forecast Outlook 2025 to 2035
Medical Device Trays Market Size and Share Forecast Outlook 2025 to 2035
Medical Adhesives Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA