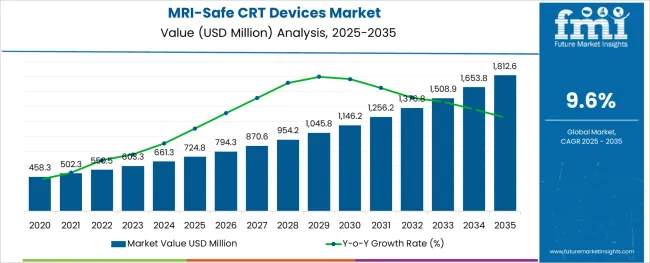
The MRI-Safe CRT Devices Market is estimated to be valued at USD 724.8 million in 2025 and is projected to reach USD 1,812.6 million by 2035, registering a compound annual growth rate (CAGR) of 9.6% over the forecast period.
The MRI safe CRT devices market is witnessing accelerated adoption due to the convergence of cardiac care advancement and imaging safety standards. As chronic heart conditions continue to rise, particularly among aging populations, the need for implantable cardiac devices that are compatible with magnetic resonance imaging has become critical. Healthcare providers are increasingly prioritizing MRI safety to avoid diagnostic delays in patients with implanted devices.
Leading manufacturers are integrating non-ferromagnetic materials, advanced shielding, and adaptive pacing algorithms that maintain device integrity during MRI exposure. Regulatory bodies and cardiology associations are also reinforcing guidelines for MRI-compatible implantables, driving wider hospital procurement and surgeon preference.
Additionally, the growing penetration of MRI scanners in secondary and tertiary care facilities is expanding patient access to advanced imaging, further encouraging the adoption of MRI safe CRT systems. With continued innovation in device miniaturization, remote programming, and condition-specific therapy, the market is expected to experience sustained growth across both developed and emerging healthcare ecosystems.
The market is segmented by Product Type, Age Group, and Indication and region. By Product Type, the market is divided into Defibrillator and Pacemaker. In terms of Age Group, the market is classified into Geriatric and Paediatric. Based on Indication, the market is segmented into Cardiomyopathy, Bradycardia, Electrocardiography, and Congestive Heart Failure. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
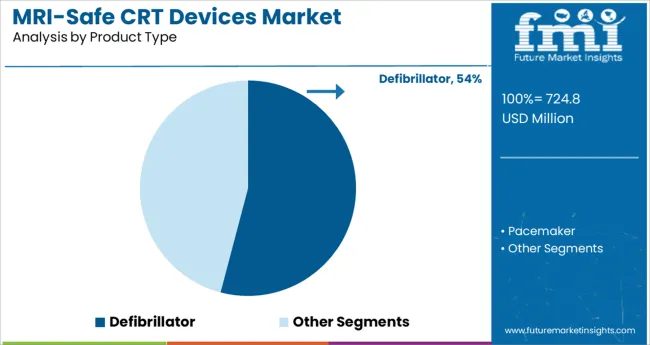
The defibrillator segment is projected to hold 54.1% of the MRI safe CRT devices market revenue in 2025, making it the leading product type. This dominance is attributed to the dual therapeutic function of defibrillation and resynchronization, particularly in high-risk heart failure patients prone to arrhythmias.
The ability of defibrillator-based CRT systems to deliver life-saving shocks while remaining safe for MRI diagnostics has made them highly favored in advanced cardiology centers. Innovations such as MRI conditional labeling, algorithm-based shock delivery, and enhanced battery longevity have reinforced clinical confidence.
Additionally, the growing incidence of ventricular arrhythmias in heart failure patients has necessitated broader deployment of CRT defibrillators. Hospitals and cardiologists are increasingly adopting these devices to avoid the trade-off between critical imaging and cardiac protection, sustaining their leadership position within the market.
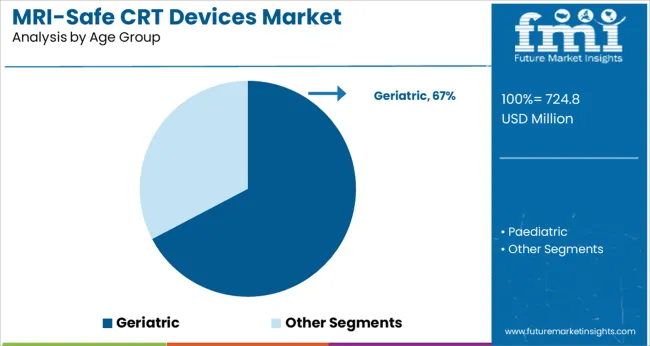
The geriatric population is expected to contribute 67.3% of the total market revenue in 2025, establishing it as the dominant age group for MRI safe CRT device implantation. This leadership is driven by the high prevalence of chronic heart failure, conduction disorders, and multi-comorbidity profiles among individuals aged 65 and above.
ge-related degeneration of the cardiac conduction system often necessitates CRT therapy, while concurrent age-linked conditions require frequent MRI-based diagnostic imaging. As a result, MRI safe devices have become essential in elderly cardiac care. Healthcare systems and cardiologists are prioritizing MRI compatible implants in older adults to minimize procedural risks and avoid future contraindications with imaging.
Moreover, extended life expectancy and increased healthcare access among geriatric populations globally are supporting volume growth. Remote monitoring features and simplified programming in these devices are also aiding adoption among aging patient groups and their care teams.
Within the indication category, cardiomyopathy is projected to account for 28.4% of market revenue in 2025, ranking it as the leading diagnostic condition driving MRI safe CRT device use. The growing global incidence of dilated and ischemic cardiomyopathy, particularly in middle-aged and elderly populations, has created heightened demand for resynchronization therapy.
MRI safe CRT devices have become critical in cardiomyopathy management due to the need for frequent cardiac MRI scans to monitor ventricular remodeling, fibrosis, and therapy response. Devices designed to remain stable under strong magnetic fields while offering optimized pacing thresholds have enabled safe longitudinal care in cardiomyopathy patients.
Additionally, clinical protocols now increasingly emphasize early CRT intervention in cardiomyopathy to improve survival and reduce hospitalization, which has propelled segmental growth. Continuous advancements in lead design, device firmware, and patient-specific programming have ensured this segment’s continued relevance and expansion.
The MRI-safe CRT devices have the ability to treat moderate to severe symptoms associated with heart failure. This kind of flexibility provided by these devices is expected to increase the MRI-safe CRT devices market size.
These devices are pocket-sized pacemakers, which are implanted just below the collarbone. This allows the patients to move around normally. Apart from that, these devices also detect heart rate abnormalities, as a result of which they transmit small pulses to synchronize the heart’s ventricles. This is expected to increase MRI-safe CRT device's market share.
A rapid increase in urbanization and improvement in healthcare infrastructure has also led to the penetration of hospitals in many parts of the world. This is expected to increase the sales of MRI-safe CRT devices. The increased urbanization has also led to an increase in disposable income, which makes MRI-safe CRT devices relatively affordable.
A number of studies have even suggested that the application of MRI-safe CRT devices has reduced the hospitalization case as well as the morbidity rate and has gradually led to an improvement in the patient’s health. This is expected to boom the demand for MRI-safe CRT devices.
There are a number of awareness programs on cardiovascular health being conducted by health regulatory bodies and governments across the world. This as well is expected to increase the sales of MRI-safe CRT devices. However, there are a few challenges that are being encountered by the MRI-safe CRT devices market. One of them is the scarcity of skilled professionals in the market.
The cost associated with these devices is also a factor that is acting as a barrier to market growth. Though there has been an increase in disposable income, not everybody can afford to buy this device.
One of the most critical barriers to the growth of the MRI-safe CRT devices market is the infection at the surgical site or the sensitivity to the device material. This is something that health professionals should take into consideration and come up with a suitable alternative.
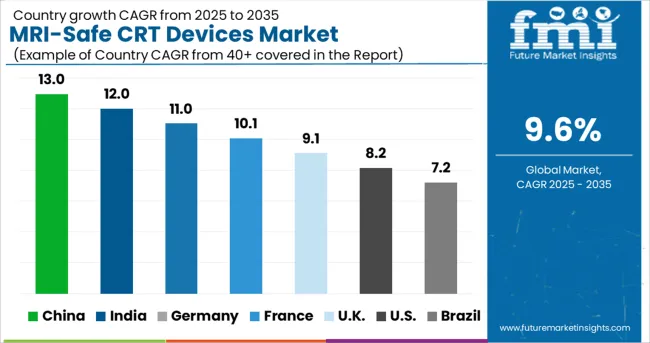
North America is currently the largest market for MRI-safe CRT devices and currently has a market share of 39.9%, and during the forecast period as well, it is expected to grow at a higher rate.
One of the major drivers of MRI-safe CRT devices in this region is the rate at which heart-based ailments are increasing amongst the masses in this region. Moreover, rapid advancements in the field of healthcare, along with relatively high disposable income, are expected to surge the adoption of MRI-safe CRT devices in North America.
Europe, which currently has a market share of 30%, is the second largest market for MRI-safe CRT devices and, during the forecast period as well, is estimated to be one of the most significant markets.
Apart from the disposable income and rapid developments in the field of healthcare, the healthcare authorities and the governments are actively spreading awareness regarding the benefits of MRI-safe CRT devices and sound cardiovascular health as well.
With a rise in heart-based ailments and even the authorities participating proactively to spread awareness regarding the same, the start-up ecosystem of the MRI-safe CRT devices market is looking for ways to provide a simple and effective solution to counter heart ailments.
One of the start-ups which are literally working towards creating an effective CRT device is ‘Cardiac Dimensions’. The Percutaneous mitral annuloplasty device leaves free access to cardiac veins for resynchronization therapy. This start-up has made it possible to implant the Carillon device prior to the CRT device in HF patients with low ejection fraction, wide QRS, and FMR.
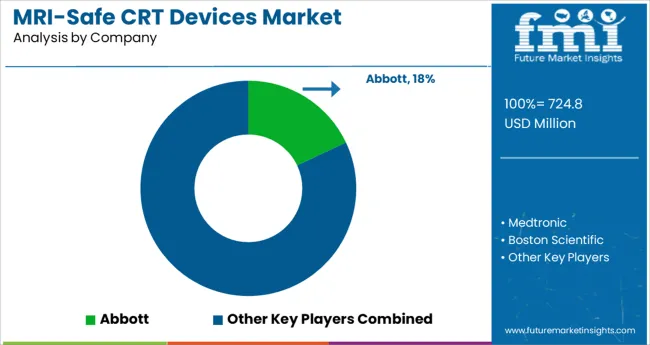
Some of the key players in the MRI-safe CRT devices market are St. Jude Medical, Abbott, Medtronic, Boston Scientific Corporation, Cook Group, Scranton Gillette Communications, Philips, LivaNova, MicroPort Scientific, BIOTRONIK, Medico, and Qinming Medical.
The key players are now developing CRT devices in such a manner that the patients will be able to track their heart rates through smartphone connectivity and connected applications. This move is expected to make the tracking process simpler.
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 9.6% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2024 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in million and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends, and Pricing Analysis |
| Segments Covered | Product Type, Age Group, Indication, Region |
| Regions Covered | North America; Latin America; Europe; East Asia; South Asia; Oceania; Middle East and Africa |
| Key Countries Profiled | United States of America, Canada, Brazil, Argentina, Germany, United Kingdom, France, Spain, Italy, Nordics, BENELUX, Australia & New Zealand, China, India, ASIAN, GCC Countries, South Africa |
| Key Companies Profiled | St. Jude Medical; Abbott; Medtronic; Boston Scientific Corporation; Cook Group, Scranton Gillette Communications; Philips; LivaNova; MicroPort Scientific; BIOTRONIK; Medico; Qinming Medical |
| Customization | Available Upon Request |
The global mri-safe crt devices market is estimated to be valued at USD 724.8 million in 2025.
It is projected to reach USD 1,812.6 million by 2035.
The market is expected to grow at a 9.6% CAGR between 2025 and 2035.
The key product types are defibrillator and pacemaker.
geriatric segment is expected to dominate with a 67.3% industry share in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
FBAR Devices Market
Snare devices Market
C-Arms Devices Market Size and Share Forecast Outlook 2025 to 2035
Timing Devices Market Analysis - Size, Growth, & Forecast Outlook 2025 to 2035
Spinal Devices Market Size and Share Forecast Outlook 2025 to 2035
Hearing Devices 3D Printing Market Size and Share Forecast Outlook 2025 to 2035
Medical Devices Market Size and Share Forecast Outlook 2025 to 2035
Network Devices Market Size and Share Forecast Outlook 2025 to 2035
Medical Devices Secondary Packaging Market Analysis by Material and Application Through 2035
Hearable Devices Market Size and Share Forecast Outlook 2025 to 2035
Lab Chip Devices Market Size and Share Forecast Outlook 2025 to 2035
Orthotic Devices, Casts and Splints Market Size and Share Forecast Outlook 2025 to 2035
Lacrimal Devices Market Size, Trends, and Forecast 2025 to 2035
Global Ablation Devices Market Trends - Growth, Innovations & Forecast 2025 to 2035
Orthotic Devices, Splints & Orthopedic Braces Market Analysis - Trends & Forecast 2024 to 2034
Ear Tube Devices Market
Pathology Devices Market Size and Share Forecast Outlook 2025 to 2035
Neurotech Devices Market Size and Share Forecast Outlook 2025 to 2035
Skin Care Devices Market Analysis - Trends & Forecast 2025 to 2035
Strapping Devices Market Trends - Size, Growth & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA