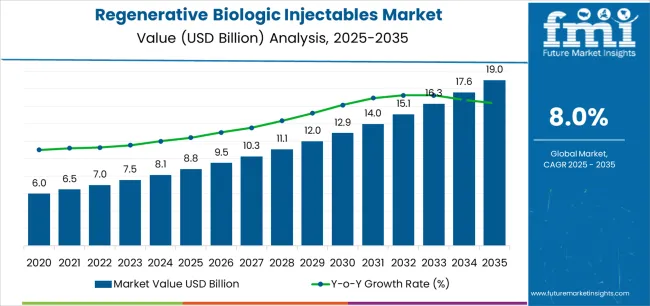
The global regenerative biologic injectables market is valued at USD 8.8 billion in 2025 and is set to reach USD 19 billion by 2035, recording an absolute increase of USD 10.2 billion over the forecast period. This translates into a total growth of 115.9%, with the market forecast to expand at a compound annual growth rate (CAGR) of 8% between 2025 and 2035. The overall market size is expected to grow by approximately 2.2X during the same period, supported by increasing demand for minimally invasive regenerative treatments, growing stem cell therapy adoption, and rising applications across sports medicine, aesthetic medicine, wound care, and specialized clinical segments.
The global regenerative biologic injectables market represents a critical segment within the regenerative medicine and biologic therapeutics industry, driven by the superior healing capabilities of biologic injectable products and the diverse functional properties of various regenerative therapy types. These specialized therapeutic products are produced through precision biotechnology processes, providing consistent clinical standards and standardized performance characteristics for various medical, aesthetic, and therapeutic applications including tissue regeneration, wound healing, and specialized biologic interventions. The processing mechanism enables controlled regenerative development, making these products particularly suitable for healthcare professionals and applications requiring specific tissue repair characteristics.
The market encompasses various product types, source origins, and specialized processing methods tailored for specific therapeutic requirements. Modern regenerative biologic injectable production incorporates advanced biotechnology, cell processing optimization, and enhanced manufacturing techniques that can deliver consistent quality across variable biologic formulations while maintaining therapeutic integrity over extended treatment periods. The integration of quality control systems, regulatory compliance protocols, and standardized processing parameters has further enhanced the value proposition of these biologic materials among healthcare manufacturers seeking reliable therapeutic performance and consistent regenerative outcomes.
Market dynamics are significantly influenced by rising regenerative medicine awareness, particularly in developed markets where personalized healthcare and advanced therapeutics drive demand for regenerative biologic injectable materials. The healthcare sector's increasing emphasis on biologic therapies, advanced regenerative formulations, and specialized tissue repair applications has created substantial demand for high-quality regenerative biologic injectable solutions in sports medicine applications, aesthetic treatment centers, and wound care facilities. Additionally, the growing trend toward precision medicine and advanced biologic technologies has amplified the need for versatile therapeutic materials capable of supporting diverse regenerative requirements and biologic medicine applications.
Consumer purchasing patterns show a marked preference for certified regenerative biologic injectable varieties that combine advanced biotechnology methods with consistent therapeutic characteristics, multiple application options, and comprehensive safety profiles for diverse clinical applications. The market has witnessed significant technological advancement in biotechnology efficiency design, quality control systems, and regulatory compliance solutions, making these products more suitable for demanding clinical environments, extended therapeutic requirements, and safety-critical regenerative operations.
Regenerative Biologic Injectables Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 8.8 billion |
| Forecast Value in (2035F) | USD 19 billion |
| Forecast CAGR (2025 to 2035) | 8% |
Between 2025 and 2030, the regenerative biologic injectables market is projected to expand from USD 8.8 billion to USD 12.9 billion, resulting in a value increase of USD 4.1 billion, which represents 40.2% of the total forecast growth for the decade. This phase of development will be shaped by increasing regenerative medicine adoption, rising demand for alternative biologic solutions, and growing availability of premium regenerative biologic injectable varieties across healthcare and aesthetic channels.
Between 2030 and 2035, the market is forecast to grow from USD 12.9 billion to USD 19 billion, adding another USD 6.1 billion, which constitutes 59.8% of the overall ten-year expansion. This period is expected to be characterized by the advancement of specialty regenerative biologic injectable applications, the development of enhanced biologic materials for therapeutic improvement, and the expansion of certified regenerative biologic injectable availability across diverse medical and aesthetic segments. The growing emphasis on biologic therapies and advanced regenerative processing will drive demand for premium regenerative biologic injectable varieties with enhanced functional properties, improved therapeutic characteristics, and superior performance profiles in specialized regenerative applications.
Between 2020 and 2024, the regenerative biologic injectables market experienced steady growth, driven by increasing awareness of regenerative medicine benefits and growing recognition of regenerative biologic injectables' effectiveness in therapeutic applications following extensive biologic medicine campaigns. The market developed as manufacturers recognized the advantages of regenerative biologic injectables over traditional therapeutic alternatives in healing-sensitive applications and began seeking specialized products designed for specific regenerative and therapeutic requirements. Technological advancement in biotechnology and regulatory compliance began emphasizing the critical importance of maintaining therapeutic safety while enhancing functional performance and improving regenerative outcomes across diverse regenerative biologic injectable applications.
From 2030 to 2035, the market is forecast to grow from USD 12.9 billion to USD 19 billion, adding another USD 6.1 billion, which constitutes 59.8% of the overall ten-year expansion. This period is expected to be characterized by the advancement of specialized processing techniques in biologic production systems, the integration of quality enhancement protocols for optimal therapeutic retention, and the development of customized biologic formulations for high-performance regenerative applications. The growing emphasis on material functionality and product reliability will drive demand for premium varieties with enhanced processing capabilities, improved storage stability, and superior therapeutic performance characteristics.
Between 2020 and 2024, the regenerative biologic injectables market experienced robust growth, driven by increasing awareness of alternative therapeutic benefits and growing recognition of specialized biologic systems' effectiveness in supporting diverse regenerative operations across medical facilities and specialty aesthetic environments. The market developed as users recognized the potential for regenerative biologic injectable products to deliver functional advantages while meeting modern requirements for biologic therapies and reliable therapeutic performance. Technological advancement in processing optimization and quality enhancement began emphasizing the critical importance of maintaining material consistency while extending product stability and improving user satisfaction across diverse regenerative biologic injectable applications.
Market expansion is being supported by the increasing global emphasis on regenerative medicine solutions and the corresponding shift toward alternative biologic systems that can provide superior therapeutic characteristics while meeting healthcare requirements for advanced regenerative solutions and cost-effective healing options. Modern healthcare manufacturers are increasingly focused on incorporating biologic materials that can enhance therapeutic performance while satisfying demands for consistent, precisely controlled regenerative development and optimized healing profiles. Regenerative biologic injectables' proven ability to deliver therapeutic excellence, functional versatility, and diverse application possibilities makes them essential materials for regenerative-focused professionals and quality-focused biologic medicine manufacturers.
The growing emphasis on biologic therapies and advanced regenerative processing is driving demand for high-performance regenerative biologic injectable systems that can support distinctive therapeutic outcomes and comprehensive regenerative benefits across sports medicine applications, aesthetic treatment centers, and specialty wound care manufacturing. Healthcare preference for biologic solutions that combine functional excellence with advanced processing methods is creating opportunities for innovative implementations in both traditional and emerging regenerative applications. The rising influence of personalized medicine and alternative therapeutic approaches is also contributing to increased adoption of regenerative biologic injectable solutions that can provide authentic functional benefits and reliable regenerative characteristics.
The market is segmented by product type, indication, source/origin, and care setting. By product type, the market is divided into platelet-rich plasma (PRP), autologous cell/BMAC/stem-cell derived, amniotic/placental allografts, and exosome/EV & other biologic. Based on indication, the market is categorized into orthopedics & MSK, aesthetics/anti-aging, wound & ulcer care, and other (neuro, uro, etc.). By source/origin, the market includes patient-derived autologous, donor allogeneic, perinatal/amniotic/placental, and engineered/cell-free exosome prep. By care setting, the market encompasses sports med/ortho clinics, aesthetic/regenerative med spa, hospital outpatient/wound centers, and pain mgmt/other clinics.
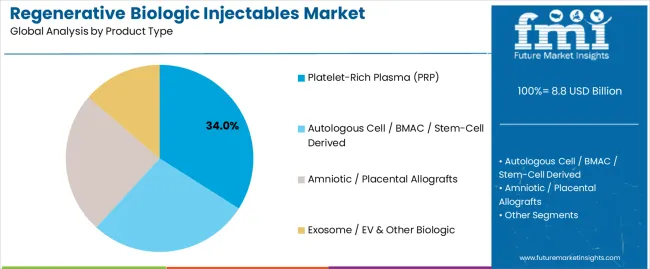
The platelet-rich plasma (PRP) segment is projected to account for 34% of the regenerative biologic injectables market in 2025, reaffirming its position as the leading product category. Healthcare manufacturers and regenerative professionals increasingly utilize PRP technologies for their superior healing characteristics, established therapeutic properties, and essential functionality in diverse regenerative applications across multiple medical sectors. PRP's proven performance characteristics and established cost-effectiveness directly address user requirements for reliable therapeutic control and optimal processing precision in sports medicine and specialty aesthetic applications.
This product segment forms the foundation of modern regenerative medicine patterns, as it represents the biologic type with the greatest therapeutic versatility and established compatibility across multiple healthcare systems. Healthcare industry investments in alternative biologic technology and regenerative optimization continue to strengthen adoption among therapeutic-focused manufacturers. With processors prioritizing biologic reliability and functional consistency, PRP systems align with both performance objectives and therapeutic requirements, making them the central component of comprehensive regenerative medicine strategies.
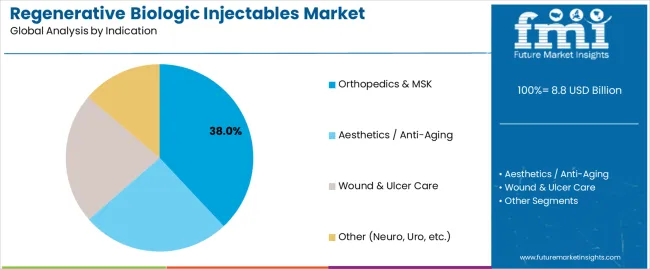
Orthopedics & MSK is projected to represent 38% of the regenerative biologic injectables market in 2025, underscoring its important role as a key indication for performance-focused users seeking superior tissue repair and enhanced therapeutic credentials. Medical facilities and treatment operations prefer orthopedics & MSK applications for their established performance characteristics, proven regenerative development, and ability to maintain exceptional therapeutic precision while supporting versatile application coverage during diverse treatment activities. Positioned as essential indications for quality-focused therapeutic processors, orthopedics & MSK offerings provide both functional excellence and therapeutic optimization advantages.
The segment is supported by continuous improvement in regenerative technology and the widespread availability of established performance standards that enable quality assurance and premium positioning at the therapeutic level. Additionally, medical facilities are optimizing biologic selections to support application-specific requirements and comprehensive therapeutic strategies. As regenerative technology continues to advance and facilities seek consistent biologic performance, orthopedics & MSK applications will continue to drive market growth while supporting operational efficiency and quality optimization strategies.
The regenerative biologic injectables market is advancing rapidly due to increasing regenerative medicine adoption and growing need for alternative biologic solutions that emphasize superior therapeutic performance across healthcare segments and specialty regenerative applications. However, the market faces challenges, including competition from other alternative therapeutic types, price volatility in raw biologic materials, and processing complexity considerations affecting development costs. Innovation in biotechnology enhancement and specialized biologic formulations continues to influence market development and expansion patterns.
Expansion of Personalized and Advanced Regenerative Applications
The growing adoption of regenerative biologic injectables with personalized certification and advanced positioning is enabling healthcare companies to develop therapeutic products that provide distinctive regenerative benefits while commanding premium pricing and enhanced healthcare appeal characteristics. Personalized applications provide superior market positioning while allowing more sophisticated product differentiation features across various therapeutic categories. Healthcare companies are increasingly recognizing the market advantages of advanced biologic positioning for comprehensive therapeutic outcomes and premium-focused regenerative marketing.
Integration of Biotechnology and Performance Enhancement Systems
Modern regenerative biologic injectable manufacturers are incorporating advanced biotechnology enhancement, performance improvement capabilities, and therapeutic supplementation systems to enhance product functionality, improve regenerative effectiveness, and meet healthcare demands for enhanced therapeutic solutions. These systems improve product performance while enabling new applications, including specialty regenerative programs and specialized therapeutic protocols. Advanced performance integration also allows manufacturers to support premium product positioning and therapeutic assurance beyond traditional biologic performance requirements.
| Country | CAGR (2025-2035) |
|---|---|
| China | 9.2% |
| Brazil | 8.8% |
| USA | 7.7% |
| UK | 7.7% |
| Germany | 7.6% |
| South Korea | 7.2% |
| Japan | 6.5% |
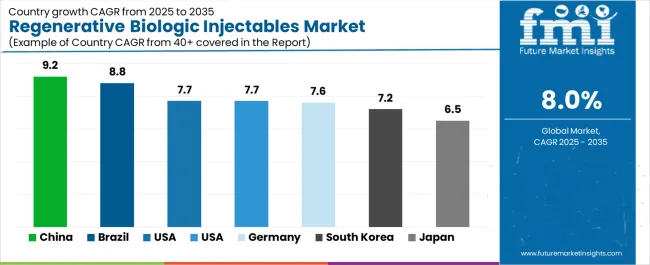
The regenerative biologic injectables market is experiencing robust growth globally, with China leading at a 9.2% CAGR through 2035, driven by the expanding regenerative medicine sector, growing biologic therapy adoption, and increasing adoption of injectable materials. Brazil follows at 8.8%, supported by rising regenerative medicine capabilities, expanding healthcare industry, and growing acceptance of biologic materials. USA shows growth at 7.7%, emphasizing established healthcare standards and comprehensive biologic development. UK records 7.7%, focusing on healthcare industry modernization and regenerative market growth. Germany demonstrates 7.6% growth, prioritizing advanced biologic technologies and therapeutic-focused healthcare products.
The report covers an in-depth analysis of 40+ countries, top-performing countries are highlighted below.
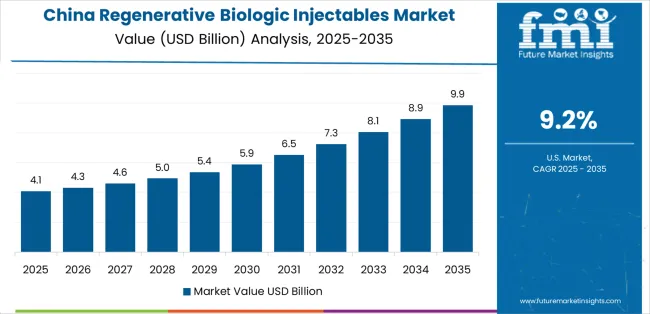
Revenue from regenerative biologic injectable consumption and sales in China is projected to exhibit exceptional growth with a CAGR of 9.2% through 2035, driven by the country's rapidly expanding regenerative medicine sector, favorable healthcare policies toward biologic therapies, and initiatives promoting injectable development across major healthcare regions. China's position as a global healthcare leader and increasing focus on processed biologic materials are creating substantial demand for high-quality regenerative biologic injectables in both domestic and international markets. Major healthcare companies and biologic distributors are establishing comprehensive injectable production capabilities to serve growing demand and emerging therapeutic opportunities.
Revenue from regenerative biologic injectable products in Brazil is expanding at a CAGR of 8.8%, supported by rising domestic healthcare consumption, growing biologic therapy adoption, and expanding therapeutic distributor capabilities. The country's developing healthcare infrastructure and increasing investment in biologic technologies are driving demand for regenerative biologic injectables across both traditional and modern healthcare applications. International biologic companies and domestic processors are establishing comprehensive operational networks to address growing market demand for alternative injectable products and efficient biologic processing solutions.
Revenue from regenerative biologic injectable products in USA is projected to grow at a CAGR of 7.7% through 2035, supported by the country's mature healthcare processing standards, established biologic therapy regulations, and leadership in specialty injectable technology. USA's sophisticated healthcare standards and strong support for alternative biologic systems are creating steady demand for both traditional and innovative injectable varieties. Leading biologic manufacturers and specialty distributors are establishing comprehensive operational strategies to serve both domestic markets and growing export opportunities.
Revenue from regenerative biologic injectable products in the UK is projected to grow at a CAGR of 7.7% through 2035, driven by the country's emphasis on healthcare industry development, biologic processing growth, and growing distributor capabilities. British manufacturers and processing facilities consistently seek quality-focused biologics that enhance product performance and support processing excellence for both traditional and modern healthcare applications. The country's position as a European healthcare leader continues to drive innovation in specialized injectable applications and healthcare processing standards.
Revenue from regenerative biologic injectable products in Germany is projected to grow at a CAGR of 7.6% through 2035, supported by established healthcare standards, mature regenerative markets, and emphasis on biologic alternatives across healthcare and aesthetic sectors. German manufacturers and specialty processors prioritize quality biologics and consistent performance, creating steady demand for premium injectable solutions. The country's comprehensive market innovation and established healthcare practices support continued development in specialized applications.
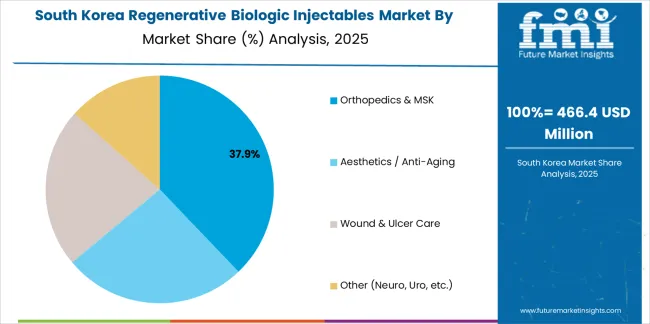
Revenue from regenerative biologic injectable products in South Korea is projected to grow at a CAGR of 7.2% through 2035, supported by established healthcare standards, mature regenerative markets, and emphasis on biologic alternatives across healthcare and aesthetic sectors. Korean manufacturers and specialty processors prioritize quality biologics and consistent performance, creating steady demand for premium injectable solutions. The country's comprehensive market maturity and established healthcare practices support continued development in specialized applications.
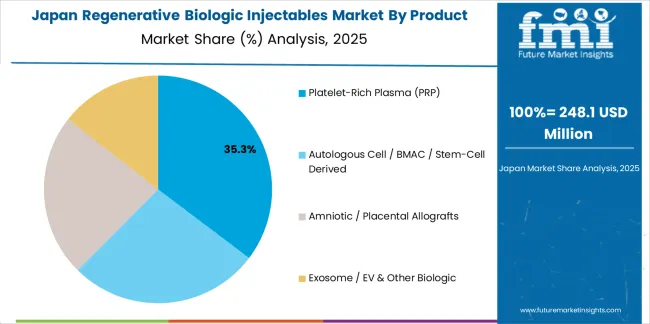
Revenue from regenerative biologic injectable products in Japan is projected to grow at a CAGR of 6.5% through 2035, supported by the country's emphasis on biologic quality, healthcare excellence, and advanced processing technology integration requiring efficient biologic solutions. Japanese healthcare facilities and quality-focused operations prioritize technical performance and regenerative precision, making specialized injectables essential materials for both traditional and modern healthcare applications. The country's comprehensive healthcare leadership and advancing quality patterns support continued market expansion.
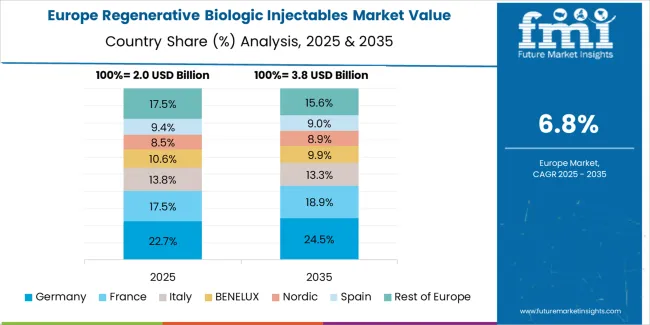
The Europe regenerative biologic injectables market is projected to grow from USD 2.64 billion in 2025 to USD 5.70 billion by 2035, registering a CAGR of 8% over the forecast period. Germany is expected to maintain its leadership position with a 28% market share in 2025, declining slightly to 27.5% by 2035, supported by its advanced healthcare infrastructure and major regenerative medicine hubs including Berlin and Munich.
France follows with a 22% share in 2025, projected to reach 22.5% by 2035, driven by comprehensive healthcare modernization programs and biologic therapy initiatives. The United Kingdom holds a 20% share in 2025, expected to decrease to 19.5% by 2035 due to market maturation. Italy commands a 15% share, while Spain accounts for 10% in 2025. The Rest of Europe region is anticipated to gain momentum, expanding its collective share from 5% to 5.5% by 2035, attributed to increasing injectable adoption in Nordic countries and emerging Eastern European markets implementing regenerative medicine programs.
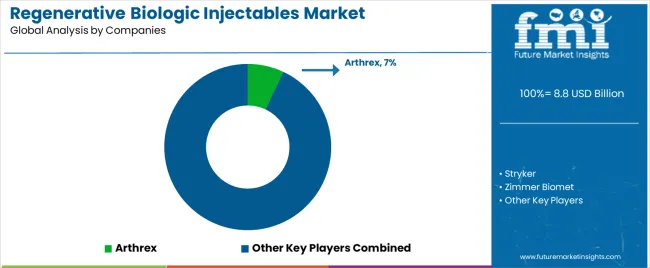
The regenerative biologic injectables market is characterized by competition among established medical device manufacturers, specialized biologic companies, and integrated regenerative medicine providers. Companies are investing in advanced biotechnologies, specialized processing engineering, product innovation capabilities, and comprehensive distribution networks to deliver consistent, high-quality, and reliable regenerative biologic injectable products. Innovation in biotechnology efficiency optimization, quality control advancement, and therapeutic-focused product development is central to strengthening market position and customer satisfaction.
Arthrex leads the market with 7% share with a strong focus on biologic technology innovation and comprehensive regenerative solutions, offering therapeutic and specialty systems with emphasis on quality excellence and regenerative heritage. Stryker provides integrated advanced biologic solutions with a focus on healthcare market applications and functional biologic networks. Zimmer Biomet delivers comprehensive injectable products with a focus on therapeutic positioning and regenerative quality. EmCyte specializes in biologic-based injectable systems with an emphasis on regenerative applications. Regen Lab focuses on comprehensive injectable processing with advanced biotechnology and therapeutic positioning capabilities. The combined other key players represent significant competition and market fragmentation among emerging biologic providers and specialized regenerative medicine companies.
Who Are the Key Players in the Regenerative Biologic Injectables Market?
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 8.8 b illion |
| Product Type | Platelet-Rich Plasma (PRP), Autologous Cell/BMAC/Stem-Cell Derived, Amniotic/Placental Allografts, Exosome/EV & Other Biologic |
| Indication | Orthopedics & MSK, Aesthetics/Anti-Aging, Wound & Ulcer Care, Other (Neuro, Uro , etc.) |
| Source/Origin | Patient-Derived Autologous, Donor Allogeneic, Perinatal/Amniotic/Placental, Engineered/Cell-Free Exosome Prep |
| Care Setting | Sports Med/Ortho Clinics, Aesthetic/Regenerative Med Spa, Hospital Outpatient/Wound Centers , Pain Mgmt /Other Clinics |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, UK, Germany, France, Japan, South Korea, China, Brazil, and 40+ countries |
| Key Companies Profiled | Arthrex , Stryker, Zimmer Biomet, EmCyte , Regen Lab, and other leading regenerative biologic injectable companies |
| Additional Attributes | Dollar sales by product type, indication, source/origin, care setting, and region; regional demand trends, competitive landscape, technological advancements in biologic processing, quality optimization initiatives, regenerative enhancement programs, and premium product development strategies |
The global regenerative biologic injectables market is estimated to be valued at USD 8.8 billion in 2025.
The market size for the regenerative biologic injectables market is projected to reach USD 19.0 billion by 2035.
The regenerative biologic injectables market is expected to grow at a 8.0% CAGR between 2025 and 2035.
The key product types in regenerative biologic injectables market are platelet-rich plasma (prp), autologous cell / bmac / stem-cell derived, amniotic / placental allografts and exosome / ev & other biologic.
In terms of indication, orthopedics & msk segment to command 38.0% share in the regenerative biologic injectables market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Regenerative Artificial Skin Market Size and Share Forecast Outlook 2025 to 2035
Regenerative Agriculture Market Size and Share Forecast Outlook 2025 to 2035
Regenerative Braking System Market Size and Share Forecast Outlook 2025 to 2035
Regenerative Turbine Pump Market Size and Share Forecast Outlook 2025 to 2035
Regenerative Medicine Market Growth – Trends & Forecast 2025 to 2035
Regenerative Blowers Market Growth – Trends & Forecast 2025 to 2035
Regenerative Thermal Oxidizer Market Growth – Trends & Forecast 2025-2035
Veterinary Regenerative Medicine Market Size and Share Forecast Outlook 2025 to 2035
Automotive Regenerative Braking Market Size and Share Forecast Outlook 2025 to 2035
Antimicrobial Regenerative Wound Matrix Market - Growth & Forecast 2025 to 2035
Biological Indicator Vial Market Size and Share Forecast Outlook 2025 to 2035
Biologics Contract Manufacturing Market Size and Share Forecast Outlook 2025 to 2035
Biologics Market Analysis - Growth & Forecast 2025 to 2035
Biological and Chemical Indicators Market Insights - Trends, Growth & Forecast 2025 to 2035
Breaking Down Market Share in Biological Indicator Vial Industry
Competitive Overview of Biologics Regulatory Affairs Market Share
Biologic Excipient Market Report – Trends, Growth & Future Outlook 2024-2034
Biologic Response Modifiers Market
Middle East & Africa (MEA) Biologics and Biosimilar Market Analysis by Drug, Drug Class, Dosage Form, Indication, Distribution Channel, and Country through 2035
Orthobiologics Market is segmented by Product Type and End User from 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA