The global 4-ethylphenylboronic acid market is valued at USD 19.7 million in 2025 and is set to reach USD 30 million by 2035, growing at a CAGR of 4.3%. The market stands at the forefront of a transformative decade that promises to redefine pharmaceutical intermediate production and fine chemical synthesis across pharmaceutical manufacturing, display materials development, and advanced chemical research sectors.
The market's journey from USD 19.7 million in 2025 to USD 30 million by 2035 represents substantial growth, demonstrating the accelerating adoption of high-purity boronic acid derivatives and sophisticated synthetic intermediates across drug discovery applications, electronic material manufacturing, healthcare compound development, and specialized chemical synthesis operations.
The first half of the decade (2025 to 2030) will witness the market climbing from USD 19.7 million to approximately USD 24.34 million, adding USD 4.62 million in value, which constitutes 45% of the total forecast growth period. This phase will be characterized by the rapid adoption of ultra-high purity systems, driven by increasing demand for pharmaceutical-grade intermediates and enhanced coupling reaction capabilities worldwide. Superior yield optimization and reaction efficiency features will become standard expectations rather than premium options.
The latter half (2030 to 2035) will witness sustained growth from USD 24.34 million to USD 30 million, representing an addition of USD 5.70 million or 55% of the decade's expansion. This period will be defined by mass market penetration of specialized pharmaceutical applications, integration with comprehensive drug development platforms, and seamless compatibility with existing synthetic chemistry infrastructure.
The market trajectory signals fundamental shifts in how pharmaceutical companies and chemical manufacturers approach intermediate synthesis, with participants positioned to benefit from sustained demand across multiple application segments.
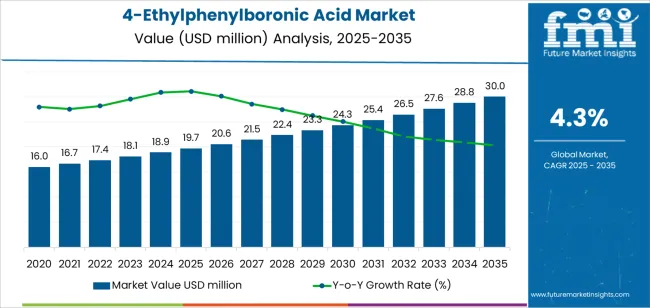
The 4-ethylphenylboronic acid market demonstrates distinct growth phases with varying market characteristics and competitive dynamics. Between 2025 and 2030, the market progresses through its pharmaceutical intermediate optimization phase, expanding from USD 19.7 million to USD 24.34 million with steady annual increments averaging 4.2% growth. This period showcases the transition from standard purity grades to ultra-high purity systems with enhanced synthetic reliability and integrated quality control becoming mainstream features.
The 2025 to 2030 phase adds USD 4.62 million to market value, representing 45% of total decade expansion. Market maturation factors include standardization of purity specifications, declining production costs for high-grade intermediates, and increasing pharmaceutical industry awareness of premium intermediate benefits reaching 85-90% effectiveness in synthetic applications. Competitive landscape evolution during this period features established manufacturers like Hebei Maison Chemical and India Fine Chemicals expanding their product portfolios while new entrants focus on specialized pharmaceutical-grade solutions and enhanced coupling reaction technology.
From 2030 to 2035, market dynamics shift toward advanced pharmaceutical integration and multi-sector deployment, with growth accelerating from USD 24.34 million to USD 30 million, adding USD 5.70 million or 55% of total expansion. This phase transition logic centers on universal high-purity systems, integration with automated synthesis equipment, and deployment across diverse pharmaceutical scenarios, becoming standard rather than specialized intermediate formats. The competitive environment matures with focus shifting from basic purity to comprehensive synthetic performance and compatibility with modern drug development operations.
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) | USD 19.7 million |
| Market Forecast (2035) | USD 30 million |
| Growth Rate | 4.30% CAGR |
| Leading Purity Grade | 99% Purity |
| Primary Application | Pharmaceutical Intermediates Segment |
The market demonstrates strong fundamentals with 99% purity systems capturing a dominant share through superior synthetic efficiency characteristics and pharmaceutical-grade manufacturing capabilities. Pharmaceutical intermediate applications drive primary demand, supported by increasing drug development requirements and enhanced coupling reaction management solutions.
Geographic expansion remains concentrated in developed pharmaceutical markets with established research infrastructure, while emerging economies show accelerating adoption rates driven by pharmaceutical manufacturing expansion and rising chemical synthesis activity.
Market expansion rests on three fundamental shifts driving adoption across pharmaceutical and chemical synthesis sectors. Pharmaceutical development growth creates compelling advantages through 4-ethylphenylboronic acid systems that provide comprehensive synthetic intermediates with standardized purity compatibility, enabling drug manufacturers to manage increasing API complexity and maintain quality standards while ensuring efficient synthetic operations and justifying investment over basic intermediate methods.
Chemical synthesis modernization accelerates as companies worldwide seek reliable intermediate systems that deliver reaction efficiency directly to synthetic operations, enabling cost reduction that aligns with pharmaceutical development expectations and maximizes synthetic pathway productivity. Drug discovery advances drive adoption from pharmaceutical consumers requiring specialized intermediate solutions that maximize coupling reaction efficiency while maintaining high-purity performance during synthesis and purification operations.
However, growth faces headwinds from raw material cost variations that differ across boronic acid suppliers regarding pricing stability and supply chain reliability, potentially limiting margin consistency in price-sensitive pharmaceutical categories. Regulatory compliance also persists regarding pharmaceutical standards and quality requirements that may increase complexity standards in markets with demanding drug development protocols.
The 4-ethylphenylboronic acid market represents a compelling intersection of pharmaceutical innovation, fine chemical advancement, and synthetic optimization management. With robust growth projected from USD 19.7 million in 2025 to USD 30 million by 2035 at a 4.30% CAGR, this market is driven by increasing pharmaceutical development expansion trends, specialty chemical requirements, and research demand for reliable intermediate formats.
The market's expansion reflects a fundamental shift in how pharmaceutical companies and chemical manufacturers approach synthetic intermediate infrastructure. Strong growth opportunities exist across diverse applications, from pharmaceutical operations requiring drug development intermediates to research facilities demanding high-purity solutions. Geographic expansion is particularly pronounced in Asia-Pacific markets, led by China (6.78% CAGR) and India (5.38% CAGR), while established markets in North America and Europe drive innovation and specialized segment development.
The dominance of 99% purity systems and pharmaceutical intermediate applications underscores the importance of proven chemical technology and synthetic reliability in driving adoption. Purity standardization and manufacturing complexity remain key challenges, creating opportunities for companies that can deliver consistent performance while maintaining cost efficiency.
Primary Classification: The market segments by purity grade into 99% purity, 98% purity, 97% purity, and research grade categories, representing the evolution from standard pharmaceutical intermediates to ultra-high purity formats for comprehensive drug development operations.
Secondary Breakdown: Application segmentation divides the market into pharmaceutical intermediates, display materials, fine chemicals, research applications, custom synthesis, and specialty chemicals sectors, reflecting distinct requirements for purity characteristics, reaction specifications, and synthetic performance.
Regional Classification: Geographic distribution covers North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa, with developed pharmaceutical markets leading innovation while emerging economies show accelerating growth patterns driven by pharmaceutical manufacturing programs.
The segmentation structure reveals technology progression from standard pharmaceutical intermediates toward integrated high-purity platforms with enhanced synthetic and reaction capabilities, while application diversity spans from drug development operations to research facilities requiring comprehensive chemical synthesis and high-purity intermediate solutions.
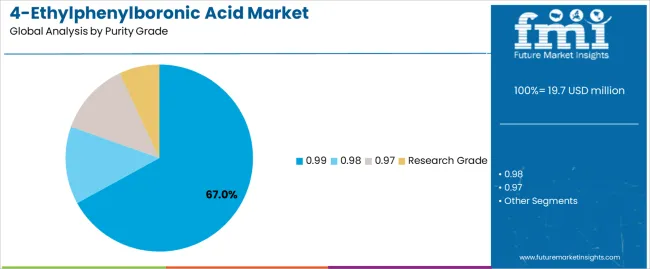
99% purity segment is estimated to account for 67% of the 4-ethylphenylboronic acid market share in 2025. The segment's leading position stems from its fundamental role as a critical component in pharmaceutical intermediate applications and its extensive use across multiple drug development and chemical synthesis sectors. 99% purity's dominance is attributed to its superior synthetic reliability, including excellent reaction efficiency, reliable coupling properties, and balanced cost-effectiveness that make it indispensable for pharmaceutical manufacturing operations.
Market Position: 99% purity systems command the leading position in the 4-ethylphenylboronic acid market through advanced purification technologies, including comprehensive quality control, uniform chemical composition, and reliable manufacturing performance that enable producers to deploy intermediate solutions across diverse pharmaceutical environments.
Value Drivers: The segment benefits from pharmaceutical preference for proven purity profiles that provide exceptional synthetic performance without requiring ultra-premium material costs. Efficient purification processes enable deployment in drug synthesis, API manufacturing, and research applications where purity reliability and cost efficiency represent critical selection requirements.
Competitive Advantages: 99% purity systems differentiate through excellent coupling reaction capacity, proven synthetic reliability, and compatibility with standard pharmaceutical equipment that enhance intermediate capabilities while maintaining economical purity profiles suitable for diverse drug development applications.
Key market characteristics:
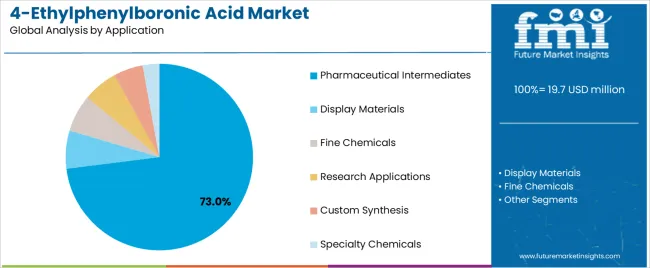
Pharmaceutical intermediates segment is projected to hold 73% of the 4-ethylphenylboronic acid market share in 2025. The segment's market leadership is driven by the extensive use of 4-ethylphenylboronic acid in drug synthesis, API development, pharmaceutical research, and chemical compound manufacturing, where boronic acids serve as both a synthetic building block and coupling solution. The pharmaceutical sector's consistent demand for reliable intermediates supports the segment's dominant position.
Market Context: Pharmaceutical intermediate applications dominate the market due to widespread adoption of efficient synthetic solutions and increasing focus on drug development, coupling reaction optimization, and synthetic pathway enhancement that support pharmaceutical manufacturing while maintaining quality standards.
Appeal Factors: Pharmaceutical consumers prioritize synthetic efficiency, coupling reliability, and integration with standard drug development systems that enable coordinated deployment across multiple synthesis needs. The segment benefits from substantial pharmaceutical growth and research development that emphasize reliable intermediate systems for drug manufacturing applications.
Growth Drivers: Drug development programs incorporate 4-ethylphenylboronic acid as standard components for pharmaceutical synthesis and API manufacturing programs. At the same time, pharmaceutical innovation initiatives are increasing demand for premium features that comply with regulatory standards and enhance synthetic performance.
Market Challenges: Raw material cost fluctuations and purity standardization may limit deployment flexibility in ultra-price-sensitive markets or regions with varying pharmaceutical requirements.
Application dynamics include:
Growth Accelerators: Pharmaceutical development expansion drives primary adoption as 4-ethylphenylboronic acid systems provide exceptional synthetic capabilities that enable drug synthesis without reaction failure, supporting pharmaceutical advancement and manufacturing efficiency that require reliable intermediate formats. Research growth accelerates market expansion as laboratories seek specialized intermediate solutions that maintain synthetic efficiency during development while enhancing reaction convenience through standardized purity and compatibility. Industry awareness increases worldwide, creating sustained demand for high-purity intermediate systems that complement research routines and provide operational advantages in synthetic pathway efficiency.
Growth Inhibitors: Raw material volatility challenges differ across chemical markets regarding price stability and supply chain consistency, which may limit margin predictability and cost planning in price-sensitive pharmaceutical categories with demanding affordability requirements. Regulatory complexity persists regarding pharmaceutical standards and quality compliance that may increase manufacturing costs in jurisdictions with strict drug development protocols. Market fragmentation across multiple purity specifications and application standards creates compatibility concerns between different synthesis systems and existing pharmaceutical infrastructure.
Market Evolution Patterns: Adoption accelerates in pharmaceutical and research sectors where synthetic benefits justify material investments, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by pharmaceutical development and chemical manufacturing expansion. Technology advancement focuses on enhanced purity properties, improved synthesis efficiency, and integration with automated pharmaceutical systems that optimize intermediate performance and production consistency. The market could face disruption if alternative synthetic intermediates or regulatory changes significantly challenge traditional 4-ethylphenylboronic acid advantages in pharmaceutical applications.
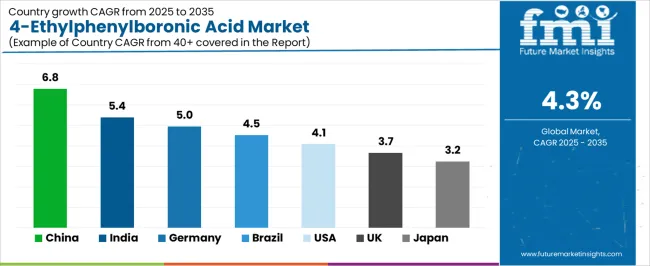
The 4-ethylphenylboronic acid market demonstrates varied regional dynamics with growth leaders including China (6.78% CAGR) and India (5.38% CAGR) driving expansion through pharmaceutical manufacturing growth and chemical synthesis modernization. Steady Performers encompass Germany (4.95% CAGR), Brazil (4.52% CAGR), and the USA (4.09% CAGR), benefiting from established pharmaceutical research systems and premium product adoption.
| Country | CAGR (2025 to 2035) |
|---|---|
| China | 6. 8% |
| India | 5.4 % |
| Germany | 5% |
| Brazil | 4.5 % |
| USA | 4.1 % |
| UK | 3.7 % |
| Japan | 3.2 % |
Regional synthesis reveals Asia-Pacific markets leading growth through pharmaceutical expansion and chemical manufacturing development, while European countries maintain steady expansion supported by specialized applications and research compliance requirements. North American markets show strong growth driven by pharmaceutical demand and research facility upgrades.
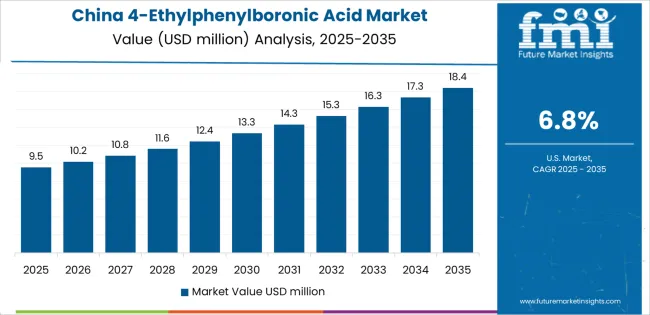
China establishes regional leadership through rapid pharmaceutical development and comprehensive chemical manufacturing modernization, integrating advanced 4-ethylphenylboronic acid systems as standard components in drug synthesis and pharmaceutical intermediate operations. The country's 6.78% CAGR through 2035 reflects pharmaceutical growth promoting chemical density and manufacturing infrastructure development that mandate the use of reliable intermediate systems in drug production operations.
Growth concentrates in major pharmaceutical centers, including Shanghai, Beijing, and Guangzhou, where manufacturing networks showcase integrated boronic acid systems that appeal to pharmaceutical companies seeking enhanced synthetic efficiency and international quality standards.
Chinese manufacturers are developing innovative 4-ethylphenylboronic acid solutions that combine local production advantages with international pharmaceutical specifications, including ultra-high purity grades and advanced coupling reaction capabilities.
Strategic Market Indicators:
The Indian market emphasizes pharmaceutical intermediate applications, including rapid chemical development and comprehensive manufacturing expansion that increasingly incorporates 4-ethylphenylboronic acid for drug synthesis and pharmaceutical production applications. The country is projected to show a 5.38% CAGR through 2035, driven by massive pharmaceutical activity under generic drug initiatives and pharmaceutical demand for standardized, high-quality intermediate systems. Indian pharmaceutical facilities prioritize cost-effectiveness with 4-ethylphenylboronic acid delivering synthetic efficiency through economical intermediate usage and reliable performance capabilities.
Technology deployment channels include major pharmaceutical companies, chemical manufacturers, and research institutions that support high-volume usage for pharmaceutical and research applications.
Performance Metrics:
The German market emphasizes advanced pharmaceutical research features, including innovative chemical technologies and integration with comprehensive drug development platforms that manage pharmaceutical synthesis, research operations, and institutional applications through unified intermediate systems. The country is projected to show a 4.95% CAGR through 2035, driven by pharmaceutical expansion under research development trends and academic demand for premium, reliable intermediate systems. German pharmaceutical institutions prioritize precision with 4-ethylphenylboronic acid delivering comprehensive synthesis through enhanced purity protection and operational innovation.
Technology deployment channels include major pharmaceutical companies, research institutions, and chemical manufacturers that support custom development for premium operations.
Performance Metrics:
In São Paulo, Rio de Janeiro, and Belo Horizonte, Brazilian pharmaceutical facilities and chemical operators are implementing advanced 4-ethylphenylboronic acid systems to enhance synthetic capabilities and support operational efficiency that aligns with pharmaceutical protocols and quality standards. The Brazilian market demonstrates sustained growth with a 4.52% CAGR through 2035, driven by pharmaceutical compliance programs and chemical investments that emphasize reliable intermediate systems for pharmaceutical and research applications.
Brazilian chemical facilities are prioritizing 4-ethylphenylboronic acid systems that provide exceptional synthetic properties while maintaining compliance with pharmaceutical standards and minimizing reaction complexity, particularly important in pharmaceutical synthesis and research facility operations.
Market expansion benefits from pharmaceutical programs that mandate enhanced intermediates in synthesis specifications, creating sustained demand across Brazil's pharmaceutical and chemical sectors, where reaction efficiency and chemical consistency represent critical requirements.
Strategic Market Indicators:
The USA market emphasizes pharmaceutical intermediate features, including innovative chemical technologies and integration with comprehensive drug development platforms that manage pharmaceutical synthesis, research operations, and commercial applications through unified intermediate systems.
The country is projected to show a 4.09% CAGR through 2035, driven by pharmaceutical expansion under drug development trends and research demand for premium, reliable intermediate systems. American pharmaceutical companies prioritize innovation with 4-ethylphenylboronic acid delivering comprehensive synthesis through enhanced coupling protection and operational advancement.
Technology deployment channels include major pharmaceutical companies, research institutions, and chemical manufacturers that support custom development for pharmaceutical operations.
Performance Metrics:
The UK market demonstrates sophisticated pharmaceutical research deployment, growing at 3.66% CAGR, with documented operational excellence in drug synthesis and research applications through integration with existing pharmaceutical systems and quality assurance infrastructure. The country leverages research expertise in pharmaceutical chemistry and intermediate production to maintain market leadership. Research centers, including London, Cambridge, and Manchester, showcase advanced installations where 4-ethylphenylboronic acid systems integrate with comprehensive pharmaceutical platforms and synthesis systems to optimize drug development and operational efficiency.
British pharmaceutical facilities prioritize synthetic precision and chemical consistency in product selection, creating demand for premium 4-ethylphenylboronic acid systems with advanced features, including ultra-high purity grades and integration with automated synthesis protocols. The market benefits from established pharmaceutical infrastructure and willingness to invest in specialized chemical technologies that provide superior synthetic properties and regulatory compliance.
Market Intelligence Brief:
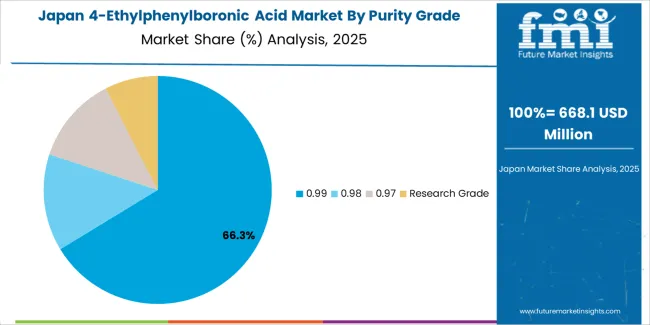
Japan's sophisticated pharmaceutical market demonstrates meticulous 4-ethylphenylboronic acid deployment, growing at 3.23% CAGR, with documented operational excellence in pharmaceutical synthesis and research applications through integration with existing drug development systems and quality assurance infrastructure. The country leverages engineering expertise in pharmaceutical manufacturing and chemical production to maintain market leadership. Pharmaceutical centers, including Tokyo, Osaka, and Yokohama, showcase advanced installations where 4-ethylphenylboronic acid systems integrate with comprehensive synthesis platforms and quality systems to optimize pharmaceutical development and operational efficiency.
Japanese pharmaceutical facilities prioritize intermediate precision and purity consistency in product selection, creating demand for premium 4-ethylphenylboronic acid systems with advanced features, including ultra-high purity grades and integration with automated pharmaceutical protocols. The market benefits from established pharmaceutical infrastructure and willingness to invest in specialized chemical technologies that provide superior synthetic properties and regulatory compliance.
Market Intelligence Brief:
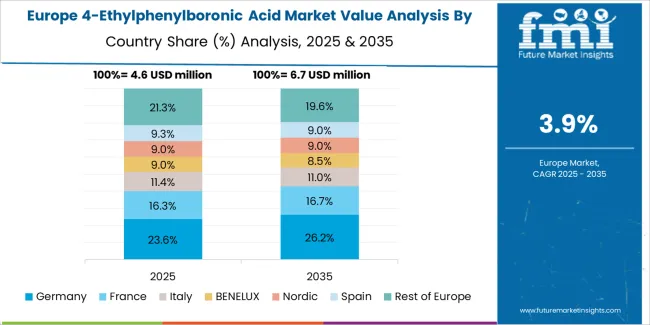
The 4-ethylphenylboronic acid market in Europe is projected to grow from USD 4.23 million in 2025 to USD 6.18 million by 2035, registering a CAGR of 3.8% over the forecast period. Germany is expected to maintain its leadership position with a 42.3% market share in 2025, declining slightly to 41.8% by 2035, supported by its pharmaceutical excellence and major research centers, including Baden-Württemberg and Bavaria.
France follows with a 19.6% share in 2025, projected to reach 20.2% by 2035, driven by comprehensive pharmaceutical programs and research facility initiatives. The United Kingdom holds a 16.8% share in 2025, expected to maintain 17.1% by 2035 through established pharmaceutical sectors and research adoption. Italy commands a 11.2% share, while Spain accounts for 6.9% in 2025. The Rest of Europe region is anticipated to gain momentum, expanding its collective share from 3.2% to 3.6% by 2035, attributed to increasing pharmaceutical development in Eastern European countries and emerging research programs implementing standardized intermediate systems.
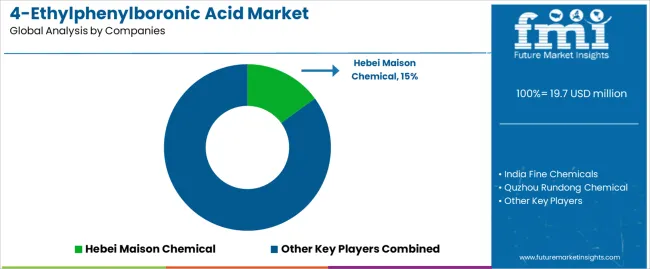
The 4-ethylphenylboronic acid market operates with moderate concentration, featuring approximately 12-18 participants, where leading companies control roughly 38-44% of the global market share through established distribution networks and comprehensive product portfolio capabilities. Competition emphasizes purity performance, manufacturing consistency, and synthetic efficiency rather than premium feature rivalry.
Market leaders encompass Hebei Maison Chemical, India Fine Chemicals, and QuzhouRundong Chemical, which maintain competitive advantages through extensive chemical manufacturing expertise, regional distribution networks, and comprehensive technical support capabilities that create pharmaceutical loyalty and support research requirements. These companies leverage decades of fine chemical production experience and ongoing purification technology investments to develop advanced 4-ethylphenylboronic acid systems with exceptional purity and synthetic features.
Specialty challengers include Nanxing Chemical and other regional manufacturers, which compete through specialized application innovation focus and efficient production solutions that appeal to pharmaceutical buyers seeking reliable performance formats and custom purity flexibility. These companies differentiate through operational efficiency emphasis and specialized market focus.
Market dynamics favor participants that combine consistent purity performance with advanced manufacturing support, including automated packaging and distribution capabilities. Competitive pressure intensifies as traditional chemical manufacturers expand into pharmaceutical intermediate systems. At the same time, specialized fine chemical producers challenge established players through innovative purification methods and cost-effective production targeting emerging pharmaceutical segments.
| Item | Value |
|---|---|
| Quantitative Units | USD 19.7 million |
| Purity Grade | 99%, 98%, 97%, Research Grade |
| Application | Pharmaceutical Intermediates, Display Materials, Fine Chemicals, Research Applications, Custom Synthesis, Specialty Chemicals |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | China, India, Germany, Brazil, USA, UK, Japan, and 25+ additional countries |
| Key Companies Profiled | Hebei Maison Chemical, India Fine Chemicals, Quzhou Rundong Chemical, Nanxing Chemical |
| Additional Attributes | Dollar sales by purity grade and application categories, regional adoption trends across Asia Pacific, North America, and Europe, competitive landscape with chemical manufacturers and pharmaceutical suppliers, customer preferences for purity characteristics and synthetic performance, integration with pharmaceutical synthesis equipment and research systems, innovations in purification technology and high-purity systems, and development of specialized intermediate solutions with enhanced coupling efficiency and pharmaceutical-grade features |
The global 4-ethylphenylboronic acid market is estimated to be valued at USD 19.7 million in 2025.
The market size for the 4-ethylphenylboronic acid market is projected to reach USD 30.0 million by 2035.
The 4-ethylphenylboronic acid market is expected to grow at a 4.3% CAGR between 2025 and 2035.
The key product types in 4-ethylphenylboronic acid market are 0.99, 0.98, 0.97 and research grade.
In terms of application, pharmaceutical intermediates segment to command 73.0% share in the 4-ethylphenylboronic acid market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Acid Coil Cleaner Market Size and Share Forecast Outlook 2025 to 2035
Acid Filling and Leveling Machine Market Size and Share Forecast Outlook 2025 to 2035
Acid Chlorides Market Size and Share Forecast Outlook 2025 to 2035
Acid-Sensitive APIs Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Acidified Whey Protein Market Analysis - Size, Share & Trends 2025 to 2035
Acid Dyes Market Growth - Trends & Forecast 2025 to 2035
Acidity Regulator Market Growth - Trends & Forecast 2025 to 2035
Acid Proof Lining Market Trends 2025 to 2035
Acid Citrate Dextrose Tube Market Trends – Growth & Industry Outlook 2024-2034
Acid Orange Market
Antacids Market Analysis – Size, Trends & Forecast 2025 to 2035
Lead Acid Battery Market Size and Share Forecast Outlook 2025 to 2035
Lead Acid Battery Recycling Market Size and Share Forecast Outlook 2025 to 2035
Feed Acidifier Market Analysis Size Share and Forecast Outlook 2025 to 2035
Food Acidulants Market Growth - Key Trends, Size & Forecast 2024 to 2034
Boric Acid Market Size and Share Forecast Outlook 2025 to 2035
Folic Acid Market Size and Share Forecast Outlook 2025 to 2035
Oleic Acid Market Size and Share Forecast Outlook 2025 to 2035
Dimer Acid-based (DABa) Polyamide Resin Market Size and Share Forecast Outlook 2025 to 2035
Humic Acid Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA