The Automated Molecular Diagnostics Testing System Market is estimated to be valued at USD 2.5 billion in 2025 and is projected to reach USD 6.1 billion by 2035, registering a compound annual growth rate (CAGR) of 9.2% over the forecast period.
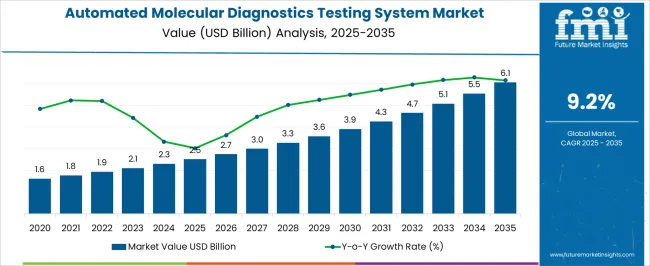
| Metric | Value |
|---|---|
| Automated Molecular Diagnostics Testing System Market Estimated Value in (2025 E) | USD 2.5 billion |
| Automated Molecular Diagnostics Testing System Market Forecast Value in (2035 F) | USD 6.1 billion |
| Forecast CAGR (2025 to 2035) | 9.2% |
The automated molecular diagnostics testing system market is witnessing strong growth momentum, driven by rising demand for rapid, accurate, and high-throughput diagnostic solutions across healthcare systems worldwide. Increasing prevalence of infectious diseases, cancer, and genetic disorders is creating a consistent need for advanced testing platforms capable of delivering precise results within shorter turnaround times. Automation is being prioritized by laboratories and hospitals to minimize human error, enhance reproducibility, and meet the growing volume of diagnostic testing.
Integration of robotics, artificial intelligence, and digital connectivity with molecular diagnostics is expanding capabilities and enabling real-time monitoring and data sharing across clinical networks. Additionally, the COVID-19 pandemic accelerated adoption of automated testing systems, establishing a long-term structural shift in diagnostics workflows.
Regulatory approvals for advanced automated solutions and growing investments in laboratory modernization are further reinforcing the market’s trajectory With the increasing importance of personalized medicine, companion diagnostics, and point-of-care testing, automated molecular diagnostic systems are anticipated to remain a cornerstone of modern healthcare, offering efficiency, accuracy, and scalability to meet evolving clinical demands.
The automated molecular diagnostics testing system market is segmented by product type, application, technique, end user, and geographic regions. By product type, automated molecular diagnostics testing system market is divided into Hardware/Instruments and Software. In terms of application, automated molecular diagnostics testing system market is classified into Oncology, Genetic Engineering, Blood Screening, Microbiology, and Others. Based on technique, automated molecular diagnostics testing system market is segmented into PCR (Polymerase Chain Reaction), FISH (Fluorescent In-situ Hybridization), Spectral Karyotype Imaging, and DNA Microarrays. By end user, automated molecular diagnostics testing system market is segmented into Hospitals, Pathology Laboratories, Research Centers, Academic Institutions, and Commercial R&D Centers. Regionally, the automated molecular diagnostics testing system industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
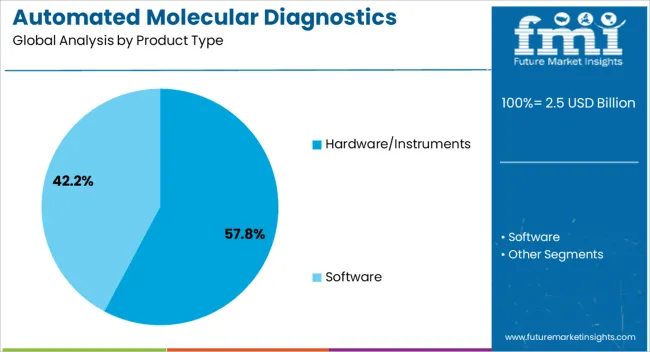
The hardware and instruments segment is projected to account for 57.8% of the automated molecular diagnostics testing system market revenue share in 2025, making it the leading product type. Its dominance is being supported by the necessity of advanced platforms that integrate robotics, precision sensors, and high-throughput systems to conduct reliable diagnostic assays. These instruments are essential for enabling automation in sample preparation, amplification, detection, and data analysis, ensuring consistency and accuracy across high testing volumes.
Hospitals, research institutions, and diagnostic laboratories are increasingly prioritizing automated hardware to minimize manual intervention, reduce operational errors, and accelerate workflow efficiency. Technological advancements are enabling compact yet multifunctional instruments that support multiple assay types, enhancing versatility for end users.
Furthermore, continuous investments by manufacturers in developing scalable, modular hardware are reinforcing adoption across large and mid-sized laboratories As healthcare systems continue to demand reliable platforms capable of handling diverse diagnostic applications with improved turnaround times, the hardware and instruments segment is expected to maintain its leadership position in the market.
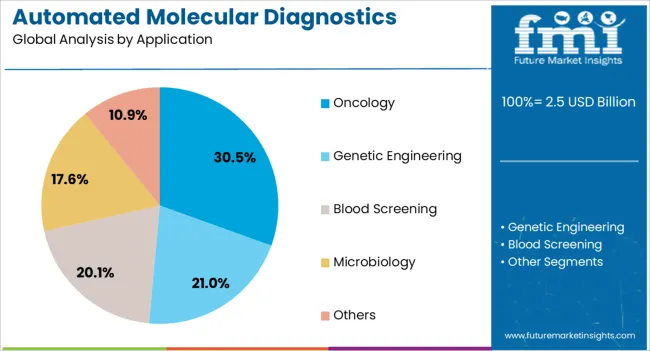
The oncology application segment is anticipated to hold 30.5% of the automated molecular diagnostics testing system market revenue share in 2025, positioning it as the leading application area. Its leadership is being driven by the growing burden of cancer globally, which has necessitated advanced diagnostic tools for early detection, staging, and monitoring of disease progression. Automated molecular testing systems are enabling precise identification of genetic mutations, biomarkers, and molecular signatures that guide targeted therapy selection and personalized treatment planning.
The ability to provide accurate, reproducible results with high sensitivity is improving clinical outcomes and supporting oncology research efforts. Growing awareness about the importance of early cancer detection, coupled with investments in companion diagnostics for immunotherapy and targeted therapy, is boosting demand for automated platforms in oncology.
Furthermore, the integration of automation allows laboratories to scale cancer testing operations efficiently while maintaining compliance with regulatory standards As cancer incidence continues to rise, oncology-focused molecular diagnostics are expected to remain the most critical driver of system adoption globally.
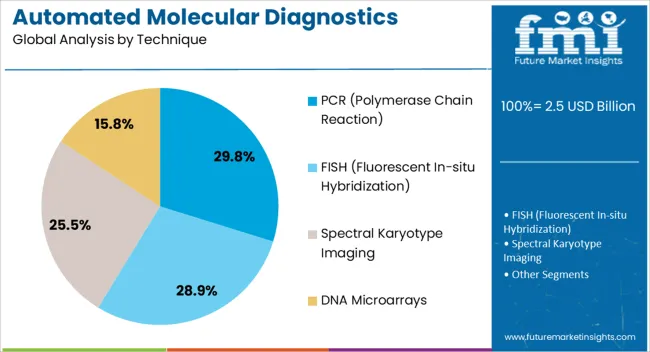
The PCR technique segment is expected to capture 29.8% of the automated molecular diagnostics testing system market revenue share in 2025, making it the leading technique. This dominance is being supported by the versatility, reliability, and accuracy of PCR in detecting a wide range of pathogens, genetic disorders, and oncology-related biomarkers. PCR’s established role in molecular diagnostics, combined with its ability to deliver high sensitivity even with low nucleic acid concentrations, is driving widespread use.
Automation has significantly enhanced PCR workflows by minimizing contamination risk, reducing turnaround times, and enabling simultaneous processing of large sample volumes. The segment is also benefiting from continuous advancements such as real-time PCR and digital PCR, which improve detection capabilities and support applications in clinical diagnostics, research, and public health.
PCR-based automated systems are being preferred due to their compatibility with existing laboratory infrastructure and regulatory validation across multiple regions With its proven reliability and adaptability to new diagnostic assays, PCR is expected to remain the cornerstone technique in automated molecular diagnostics testing.
The technological developments in genetic engineering has created many new aspects in diagnostic sciences. The molecular diagnostic techniques are used for understanding the biological samples and their genetic code and figure out the irregularities caused in an individual's DNA.
The molecular diagnostic involves various techniques which are time consuming, costly and require expertise to perform. These techniques are also subjected to errors which may lead to wrong interpretation of data and loss of valuable test materials and time. To compensate this loss of time and money automated molecular diagnostic systems are required.
These systems run on computerized programs to carry out diagnostic processes on a given biological sample. These systems are highly effective and accurate. With the changing trend towards personalized medicine and developments in genetic engineering the automated molecular diagnostics testing system market is expected to boost during the forecast period.
The major drivers of automated molecular diagnostic system market are increasing demand for more number of healthcare facilities and diagnostics laboratories along with high adoption rate of automated molecular diagnostics testing systems.
The introduction of cloud based databases that are used by the automated molecular diagnostic testing systems to determine the samples is providing significant growth opportunities to automated molecular diagnostic testing system market.
The increasing demand of molecular diagnostics requires low time consuming techniques, this is providing traction for automated molecular diagnostics testing systems market. Along with low time cycle automated systems are also able to provide more information on samples and analyze multiple organisms for detection of infectious diseases.
However the high price of these systems and requirement of the highly skilled labor to use these systems is currently hampering the growth of automated molecular diagnostic testing systems market.
With the increasing demand for the automated molecular diagnostics testing systems and high cost of molecular diagnostics tests, several companies are trying to develop effective automated and self-contained, small sized analyzers at low cost. Also companies are trying to develop efficient and user friendly databases for automated systems
Geographically the automated molecular diagnostics testing system market is segmented into five regions: North America, Latin America, Europe, Asia Pacific and MEA region. North America and Europe are leading region in the automated molecular diagnostic market mainly due to higher adoption rate towards automation in the healthcare industry.
Also the rate of healthcare expenditure in North America and Europe is high along with demand for latest technology. The adoption rate of personalized medicine is also very high in the USA and EU5 countries which is boosting the automated molecular diagnostic testing market. APAC region is expected exhibit significant growth in terms of revenue over the forecast period.
The large number of research centers in China and India and R&D centers in Japan will lead to rise in demand for high quality of molecular diagnostics which is ultimately expected to boost the demand of automated molecular diagnostic testing systems in the APAC region during the forecast period.
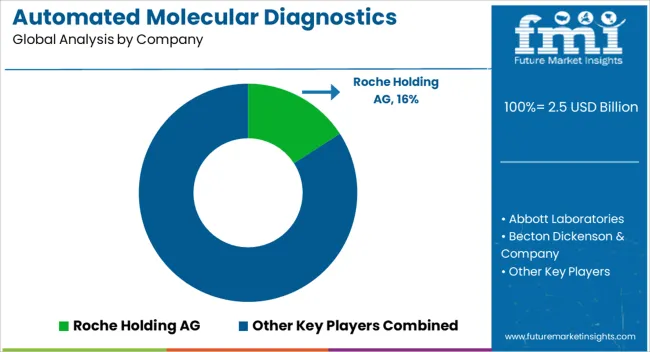
Some key participants in the automated molecular diagnostic market are Roche Holding AG, Abbott Laboratories, Becton Dickenson & Company, Siemens Healthcare GmbH, Enigma Diagnostics Ltd., Qiagen, Bio-Rad Laboratories, Inc., Cepheid Inc. and PerkinElmer Inc., AutoGenomics, ELITech Group.
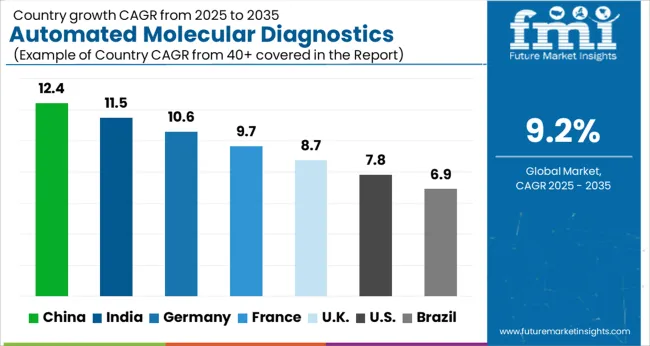
| Country | CAGR |
|---|---|
| China | 12.4% |
| India | 11.5% |
| Germany | 10.6% |
| France | 9.7% |
| UK | 8.7% |
| USA | 7.8% |
| Brazil | 6.9% |
The Automated Molecular Diagnostics Testing System Market is expected to register a CAGR of 9.2% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 12.4%, followed by India at 11.5%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 6.9%, yet still underscores a broadly positive trajectory for the global Automated Molecular Diagnostics Testing System Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 10.6%. The USA Automated Molecular Diagnostics Testing System Market is estimated to be valued at USD 887.4 million in 2025 and is anticipated to reach a valuation of USD 1.9 billion by 2035. Sales are projected to rise at a CAGR of 7.8% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 119.8 million and USD 63.3 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 2.5 Billion |
| Product Type | Hardware/Instruments and Software |
| Application | Oncology, Genetic Engineering, Blood Screening, Microbiology, and Others |
| Technique | PCR (Polymerase Chain Reaction), FISH (Fluorescent In-situ Hybridization), Spectral Karyotype Imaging, and DNA Microarrays |
| End User | Hospitals, Pathology Laboratories, Research Centers, Academic Institutions, and Commercial R&D Centers |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
| Key Companies Profiled | Roche Holding AG, Abbott Laboratories, Becton Dickenson & Company, Siemens Healthcare GmbH, Enigma Diagnostics Ltd, Qiagen, Bio-Rad Laboratories, Cepheid Inc., PerkinElmer Inc., AutoGenomics, and ELITech Group |
The global automated molecular diagnostics testing system market is estimated to be valued at USD 2.5 billion in 2025.
The market size for the automated molecular diagnostics testing system market is projected to reach USD 6.1 billion by 2035.
The automated molecular diagnostics testing system market is expected to grow at a 9.2% CAGR between 2025 and 2035.
The key product types in automated molecular diagnostics testing system market are hardware/instruments and software.
In terms of application, oncology segment to command 30.5% share in the automated molecular diagnostics testing system market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Automated Radionuclide Dispenser Market Size and Share Forecast Outlook 2025 to 2035
Automated Test Equipment Market Size and Share Forecast Outlook 2025 to 2035
Automated Machine Learning Market Forecast Outlook 2025 to 2035
Automated CPR Device Market Size and Share Forecast Outlook 2025 to 2035
Automated Compound Storage and Retrieval (ACSR) Market Size and Share Forecast Outlook 2025 to 2035
Automated People Mover Market Size and Share Forecast Outlook 2025 to 2035
Automated Infrastructure Management Solution Market Size and Share Forecast Outlook 2025 to 2035
Automated Mineralogy Solution Market Size and Share Forecast Outlook 2025 to 2035
Automated Material Handling Equipment Market Size and Share Forecast Outlook 2025 to 2035
Automated Labeling Machines Market Size and Share Forecast Outlook 2025 to 2035
Automated Solar Panel Cleaning Market Size and Share Forecast Outlook 2025 to 2035
Automated Infrastructure Management (AIM) Solutions Market Size and Share Forecast Outlook 2025 to 2035
Automated Window Blinds Market Size and Share Forecast Outlook 2025 to 2035
Automated Algo Trading Market Size and Share Forecast Outlook 2025 to 2035
Automated Number Plate Recognition (ANPR) and Detection Sensors Market Size and Share Forecast Outlook 2025 to 2035
Automated Tray Fill and Seal Machines Market Size and Share Forecast Outlook 2025 to 2035
Automated Poly Bagging Machines Market Size and Share Forecast Outlook 2025 to 2035
Automated External Defibrillator Market Analysis – Size, Share, and Forecast 2025 to 2035
Automated Suturing Devices Market Analysis - Growth & Forecast 2025 to 2035
Automated Blood Processing Equipment Market Growth – Trends & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA