The Beta Thalassemia Testing Market is estimated to be valued at USD 564.4 million in 2025 and is projected to reach USD 1241.3 million by 2035, registering a compound annual growth rate (CAGR) of 8.2% over the forecast period.
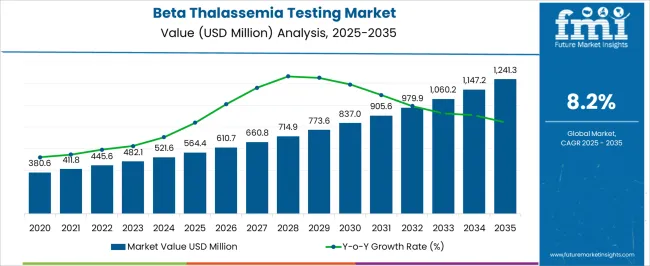
| Metric | Value |
|---|---|
| Beta Thalassemia Testing Market Estimated Value in (2025 E) | USD 564.4 million |
| Beta Thalassemia Testing Market Forecast Value in (2035 F) | USD 1241.3 million |
| Forecast CAGR (2025 to 2035) | 8.2% |
The beta thalassemia testing market is expanding steadily due to the growing need for early and precise genetic screening to manage inherited hemoglobin disorders. Increased global carrier prevalence, especially in regions with high consanguinity rates, has emphasized the importance of accurate diagnostics.
Advancements in genomic technologies and the integration of population screening programs have facilitated broader adoption of high throughput testing methods. Supportive government policies, prenatal screening mandates, and rising awareness about genetic counseling have also accelerated market demand.
The move toward personalized medicine and preventive healthcare has positioned beta thalassemia testing as a critical component in reproductive and neonatal health management. The future outlook remains promising, driven by technological innovation, cost reduction in sequencing platforms, and expanding access to advanced molecular diagnostics across public and private healthcare infrastructures.
The market is segmented by Technology, Test Type, Setting, and End-User and region. By Technology, the market is divided into Next-Generation Sequencing, Polymerase Chain Reaction, and Others. In terms of Test Type, the market is classified into Prenatal Testing, Genetic Testing, and Others. Based on Setting, the market is segmented into Laboratories and Point of Care. By End-User, the market is divided into Hospitals & Clinics, Clinical Laboratories, and Others. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
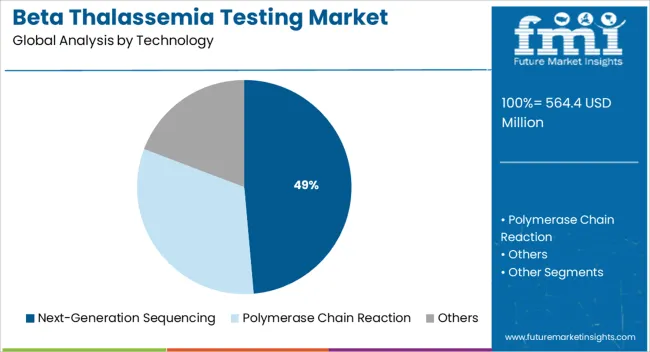
The next generation sequencing segment is expected to contribute 48.60% of the total market revenue by 2025, establishing it as the most dominant technology category. This growth is fueled by the method’s ability to detect a wide range of mutations with high accuracy and throughput.
It enables comprehensive screening of beta globin gene clusters and supports multiplex analysis, offering a more detailed genetic profile in a single test run. The scalability of next generation sequencing along with declining costs per sample and improved turnaround times has made it suitable for both population based carrier screening and diagnostic applications.
Its capability to identify rare and novel mutations has further reinforced its position as the preferred technology for beta thalassemia testing in clinical genomics laboratories.
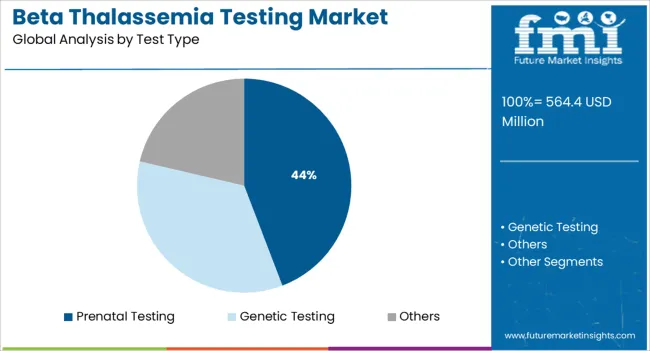
The prenatal testing segment is projected to hold 44.20% of total market share by 2025, making it the leading test type in the market. This dominance is driven by the growing demand for early detection of inherited genetic disorders in the fetus, especially among high risk pregnancies.
Prenatal screening for beta thalassemia enables informed reproductive choices and early intervention strategies. Increasing awareness around carrier status and government backed screening programs have made prenatal testing more accessible and routine in maternity care.
Non invasive techniques combined with molecular methods have also improved safety and accuracy, contributing to the segment’s prominence. This test type continues to lead due to its essential role in preventive genetic counseling and risk assessment.
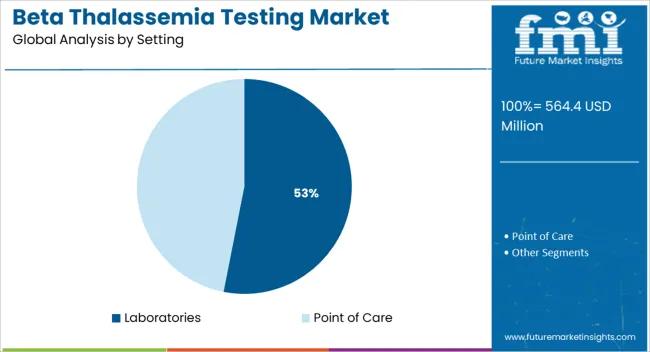
The laboratories segment is anticipated to account for 53.10% of the total revenue by 2025 within the setting category, positioning it as the most significant service environment. Laboratories offer the infrastructure and technical expertise required for complex genetiC testing such as next generation sequencing and molecular diagnostics.
Their capacity for high volume sample processing, quality control, and result interpretation supports accurate diagnosis and reliable reporting. Centralized testing facilities are often preferred for confirmatory testing, carrier screening, and prenatal diagnostics due to their advanced equipment and standardized procedures.
The continued reliance on laboratories for comprehensive thalassemia testing reflects their vital role in delivering clinically validated, regulated, and high complexity diagnostic services.
The market for beta thalassemia testing is anticipated to develop significantly throughout the projected period as a result of rising venous disease prevalence and rising new product approvals to meet this demand.
The market is expected to develop as a result of rising disease awareness, the growing value of early detection, and rising spending on chronic illnesses. Beta thalassemia testing products are being used more frequently in both developed and developing nations due to the development of technologically sophisticated products.
An important element driving the market is the rapid uptake of improved technologies for early diagnosis and treatment, which has increased the number of diagnostic tests performed in both private and public laboratories.
To take advantage of the enormous untapped market prospects in the developing world, the market's top companies are concentrating on creating products that are both affordable and of excellent quality. The rising expenditures on R&D and the rising number of new product approvals provide evidence of this.
Beta thalassemia testing products are not widely adopted in poorer countries around the world because of a lack of favourable insurance systems, insufficient knowledge of the disease, high product costs, and poor economic conditions.
The Beta thalassemia testing market participants may benefit from significant growth potential by introducing new, low-cost goods and making more attempts to penetrate these markets.
North America is likely to dominate the global market for beta thalassemia testing
A sizable portion of the market for beta thalassemia testing globally is anticipated to come from North America. The presence of significant market participants and developed healthcare infrastructure in the area is credited with driving the market growth.
The Asia Pacific beta thalassemia testing market is anticipated to grow quickly over the course of the forecast period. Major factors that are predicted to boost the market include increased awareness of the need for early diagnosis and an increase in the number of beta thalassemia cases.
The global beta thalassemia testing market is estimated to be valued at USD 564.4 million in 2025.
The market size for the beta thalassemia testing market is projected to reach USD 1,241.3 million by 2035.
The beta thalassemia testing market is expected to grow at a 8.2% CAGR between 2025 and 2035.
The key product types in beta thalassemia testing market are next-generation sequencing, polymerase chain reaction and others.
In terms of test type, prenatal testing segment to command 44.2% share in the beta thalassemia testing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Beta-Glucanase Market Size and Share Forecast Outlook 2025 to 2035
Beta-Glucan-Based Actives Market Analysis - Size and Share Forecast Outlook 2025 to 2035
Beta Carotene Market Analysis - Size, Share, and Forecast 2025 to 2035
Beta-Glucans Market Trends – Growth, Demand & Forecast 2025 to 2035
Betaine Market Analysis - Product Innovations & Market Trends
Betanin Food Color Market Insights - Natural Pigments & Industry Demand 2024 to 2034
Beta-Alanine Market
Beta-Hydroxybutyrate Testing Market
Beta thalassemia Treatment Market
Abetalipoproteinemia Management Market Size and Share Forecast Outlook 2025 to 2035
Oat Beta-Glucan Market Analysis - Size, Share, and Forecast 2025 to 2035
Yeast Beta Glucan Market Size and Share Forecast Outlook 2025 to 2035
Long-Acting Beta-Agonists Market Size and Share Forecast Outlook 2025 to 2035
Short-Acting Beta-Agonists Market – Trends, Demand & Forecast 2025 to 2035
Testing, Inspection & Certification Market Growth – Trends & Forecast 2025 to 2035
5G Testing Market Size and Share Forecast Outlook 2025 to 2035
AB Testing Software Market Size and Share Forecast Outlook 2025 to 2035
5G Testing Equipment Market Analysis - Size, Growth, and Forecast 2025 to 2035
Eye Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HSV Testing Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA