The Endovascular Therapy Devices Market is estimated to be valued at USD 4.8 billion in 2025 and is projected to reach USD 9.3 billion by 2035, registering a compound annual growth rate (CAGR) of 6.8% over the forecast period.
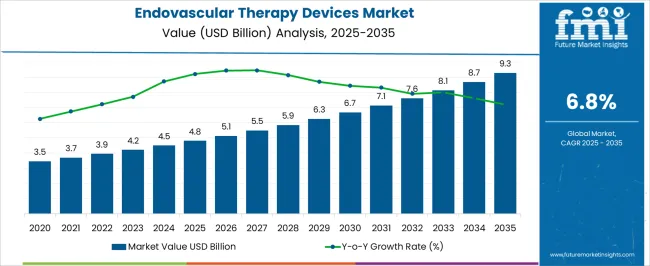
| Metric | Value |
|---|---|
| Endovascular Therapy Devices Market Estimated Value in (2025 E) | USD 4.8 billion |
| Endovascular Therapy Devices Market Forecast Value in (2035 F) | USD 9.3 billion |
| Forecast CAGR (2025 to 2035) | 6.8% |
The Endovascular Therapy Devices market is witnessing strong growth driven by the increasing prevalence of cardiovascular diseases, peripheral artery disease, and vascular disorders. The market is being shaped by the demand for minimally invasive treatment options that reduce patient recovery time, procedural risks, and hospital stays. Advancements in device design, imaging technologies, and procedural techniques are enabling higher precision and improved patient outcomes.
Growing adoption of endovascular interventions over traditional open surgery is contributing to market expansion, particularly in developed regions with advanced healthcare infrastructure. Rising awareness among physicians and patients about the benefits of minimally invasive therapies, coupled with an aging global population, is supporting sustained demand. Investments in R&D for innovative grafts, stents, and navigation systems are also facilitating the development of safer and more effective devices.
In addition, increased availability of reimbursement programs and hospital preference for cost-effective treatment methods are driving adoption The market outlook is favorable, with significant opportunities expected in emerging economies as healthcare access expands and advanced treatment options become more widely available.
The endovascular therapy devices market is segmented by product type, procedures, application, end user type, and geographic regions. By product type, endovascular therapy devices market is divided into Percutaneous endovascular aneurysm repair (EVAR), Fenestrated EVAR, Aortic stents, Biodegradable Stents, Self-expanding Nitinol Stents, Thoracic aortic aneurysms grafts, and Other Devices. In terms of procedures, endovascular therapy devices market is classified into Angioplasty with Stent Placement, Balloon Angioplasty, Drug-Eluting Stents (DES), Renal Artery Angioplasty and Stenting, Carotid Artery Stenting, Transfemoral Carotid Artery Stenting, Transcarotid Artery Revascularization, Intravascular Brachytherapy, Atherectomy, and Thrombolysis. Based on application, endovascular therapy devices market is segmented into Cardiology, Pulmonary, Vascular, Surgery, Neurology, and Radiology. By end user type, endovascular therapy devices market is segmented into Hospitals, Clinics, and Research Institutes. Regionally, the endovascular therapy devices industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
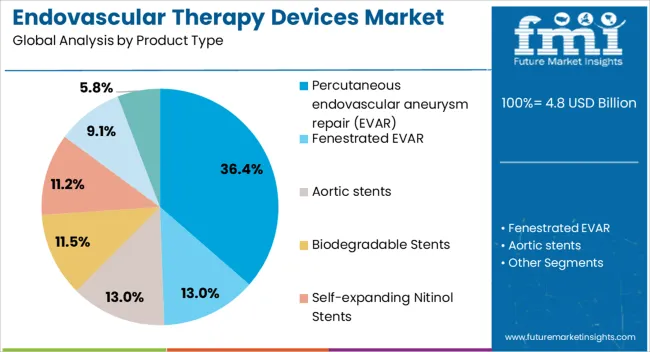
The Percutaneous Endovascular Aneurysm Repair EVAR segment is projected to account for 36.40% of the Endovascular Therapy Devices market revenue in 2025, establishing it as the leading product type. This prominence is being attributed to the minimally invasive nature of EVAR procedures, which reduces procedural risk, hospital stay, and recovery time compared to open surgical approaches. Adoption has been supported by improved device designs, including flexible stent grafts and enhanced imaging systems, which facilitate precise deployment in complex vascular anatomies.
The segment has been further strengthened by increasing clinical preference for outpatient and day-surgery procedures, as well as growing patient awareness of less invasive treatment options. Hospitals and healthcare providers are prioritizing EVAR due to its potential to reduce postoperative complications and associated costs.
Additionally, ongoing innovations in endovascular navigation and delivery systems are enabling broader applicability of EVAR in challenging cases As patient demand for safer and faster recovery treatments rises, the EVAR segment is expected to maintain its leading position, driven by procedural efficiency and favorable clinical outcomes.
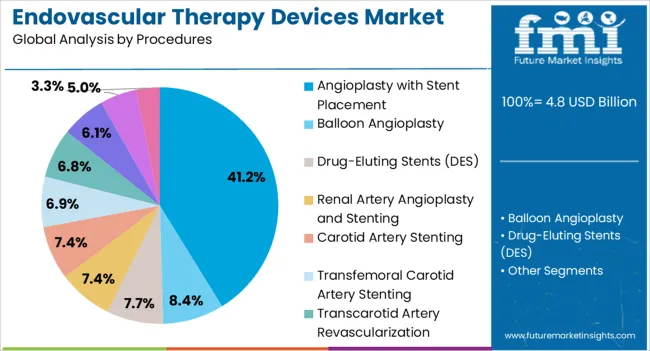
The Angioplasty with Stent Placement segment is expected to hold 41.20% of the market revenue in 2025, making it the dominant procedural category in the Endovascular Therapy Devices market. This leadership is being supported by the widespread prevalence of coronary artery disease and peripheral arterial disease, which necessitate rapid and effective intervention. The minimally invasive nature of angioplasty combined with stent placement ensures immediate restoration of vessel patency, improved blood flow, and reduced procedural morbidity.
Adoption has been facilitated by advancements in stent technology, including drug-eluting stents, bioresorbable scaffolds, and improved delivery systems, which increase procedural success rates and long-term outcomes. Healthcare providers are increasingly preferring angioplasty with stent placement due to its cost-effectiveness and shorter recovery periods, allowing faster patient throughput.
Additionally, clinical guidelines favoring early intervention in high-risk patients and improved imaging modalities have accelerated the adoption of this procedure The combination of proven efficacy, patient demand, and continual innovation is expected to sustain its dominant market share in the coming years.
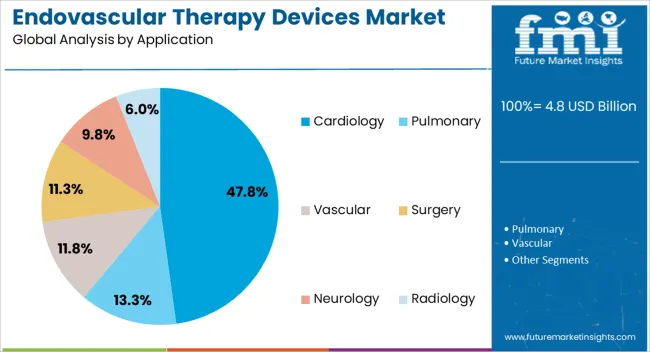
The Cardiology end-use segment is projected to account for 47.80% of the Endovascular Therapy Devices market revenue in 2025, establishing it as the leading industry application. Growth in this segment is being driven by the high prevalence of cardiovascular diseases worldwide, including coronary artery disease, aneurysms, and heart valve disorders, which require endovascular intervention. The adoption of minimally invasive therapies in cardiology has been accelerated by technological advancements in imaging, catheter-based devices, and precision navigation systems.
Hospitals and clinics are increasingly prioritizing cardiology-focused endovascular procedures to reduce patient risk, minimize hospital stays, and improve clinical outcomes. Additionally, aging populations, rising patient awareness, and favorable insurance coverage for cardiac interventions are contributing to high demand.
Continuous innovation in stents, grafts, and catheter-based technologies enables cardiologists to treat complex cases more effectively, further reinforcing the segment's dominance As cardiovascular disease remains a leading cause of morbidity and mortality globally, the cardiology application is expected to continue driving revenue growth in the Endovascular Therapy Devices market.
A procedure involving endovascular therapy devices are used to insert a flexible, sterile plastic tube, stents or a catheter into a blood vessel to allow blood to be withdrawn from or medication to be delivered to a patient's bloodstream over a period of time. These endovascular therapy devices can be used either for intravenous antibiotic treatment, chemotherapy, long-term intravenous feeding and blood transfusions.
The market is challenged by many medical complexities requiring cardiovascular disease treatment from inside the blood vessel, strokes and other cardiovascular conditions. The market of endovascular therapy devices contains products which are less invasive, requiring patients to spend less time in the hospital, less recovery time and devices which cause less pain. The endovascular therapy devices market is dominated by minimally invasive, catheter-based procedure. These procedures are often performed in a cardiac catheterization labs present in hospitals.
The global endovascular therapy devices market will increase in forecast period 2025-28. Endovascular therapy devices market will be driven primarily by the rise in incidence of cardiovascular patients, economic power in some regions leading to lifestyle change, growing disposable income and, increasing expenditure.
In addition, recent developments in stent technology and increased experience of interventionists will propel the growth of the global endovascular therapy devices market in the future. However, high cost involved in usage of new endovascular therapy devices, less awareness in economically poor nations, and Us's stringent tax regulation rules on medical devices are expected to be some of the major restraints in global market for endovascular therapy devices market.
The endovascular therapy devices market has grown exponentially since its inception in the early 1990s. Global estimates for the market reflects several factors. The use of manual compression has significantly lowered down in obese patients or patients on anticoagulation. However, complex procedures performed with a percutaneous endovascular therapy devices have taken strides as they offer bigger sheath sizes. As the economic landscape of health care becomes more competitive, endovascular therapy devices are becoming increasingly valuable because it frees up time for other work.
Current market trends are focused on developing endovascular therapy devices that can be safe and efficient enough to close large-caliber femoral arteriotomies. As transcatheter aortic valve repair and percutaneous endovascular aneurysm repair are increasing in popularity, there will be tremendous opportunity for the manufacturers in the market to develop and manufacture simple but effective endovascular therapy devices in future.
Globally, global Endovascular therapy Devices market is segmented into seven key regions viz. North America, Latin America, Western Europe, Eastern Europe, Asia Pacific excluding Japan, Japan, and Middle East. North America will account for the largest share in global Endovascular therapy Devices market. Besides the region's Endovascular therapy Devices market growth will also increase due to obesity and diet, and new products lunch due to presence of leading manufacturers in the region. Europe with robust healthcare infrastructure, will drive the endovascular therapy devices market in this region. Technological advances in the region in the past decade have shifted revascularization strategies from traditional open surgical approaches.
Focus has shifted towards lower-morbidity percutaneous endovascular treatments for patients with peripheral arterial disease. Asia Pacific mainly including India, China, AUS&NZ and Latin American countries are expected to be a significant market contributors in the rising Endovascular therapy Devices market. Advancements in stent designs by leading and local manufacturers in the region have fueled the growth of endovascular therapy devices in the region due liberalization of healthcare polices and trade related implications.
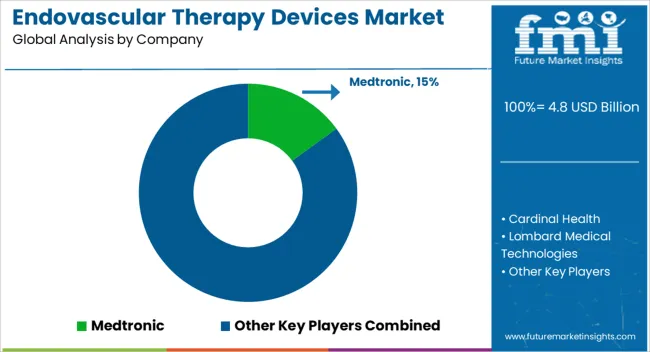
Some of the key players present in global Endovascular therapy Devices market include Cardinal Health, Altura Medical, Lifetech Scientific BiFlow Medical, Boston Scientific, Endospan, Getinge, InspireMD, Japan Lifeline, Lombard Medical Technologies, Endologix, Medtronic, Nellix, Penumbra and Terumo. In addition presence of small and local manufacturers across the countries will account for competiveness in Endovascular therapy Devices market.
The report is a compilation of first-hand information, qualitative and quantitative assessment by industry analysts, inputs from industry experts and industry participants across the value chain. The report provides in-depth analysis of parent market trends, macroeconomic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.
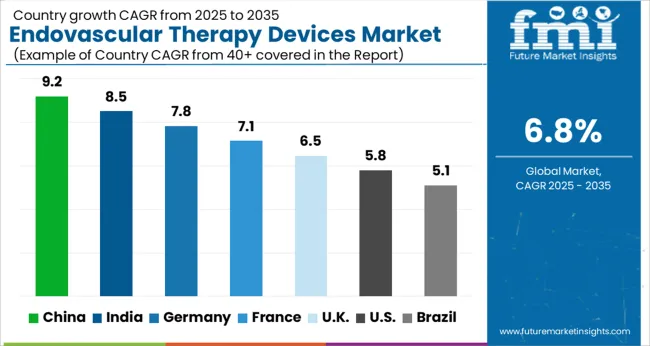
| Country | CAGR |
|---|---|
| China | 9.2% |
| India | 8.5% |
| Germany | 7.8% |
| France | 7.1% |
| UK | 6.5% |
| USA | 5.8% |
| Brazil | 5.1% |
The Endovascular Therapy Devices Market is expected to register a CAGR of 6.8% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 9.2%, followed by India at 8.5%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 5.1%, yet still underscores a broadly positive trajectory for the global Endovascular Therapy Devices Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 7.8%. The USA Endovascular Therapy Devices Market is estimated to be valued at USD 1.7 billion in 2025 and is anticipated to reach a valuation of USD 2.9 billion by 2035. Sales are projected to rise at a CAGR of 5.8% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 237.5 million and USD 122.1 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 4.8 Billion |
| Product Type | Percutaneous endovascular aneurysm repair (EVAR), Fenestrated EVAR, Aortic stents, Biodegradable Stents, Self-expanding Nitinol Stents, Thoracic aortic aneurysms grafts, and Other Devices |
| Procedures | Angioplasty with Stent Placement, Balloon Angioplasty, Drug-Eluting Stents (DES), Renal Artery Angioplasty and Stenting, Carotid Artery Stenting, Transfemoral Carotid Artery Stenting, Transcarotid Artery Revascularization, Intravascular Brachytherapy, Atherectomy, and Thrombolysis |
| Application | Cardiology, Pulmonary, Vascular, Surgery, Neurology, and Radiology |
| End User Type | Hospitals, Clinics, and Research Institutes |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
| Key Companies Profiled | Medtronic, Cardinal Health, Lombard Medical Technologies, Lifetech Scientific, Boston Scientific, Endospan, Getinge, InspireMD, Japan Lifeline, Endologix, Penumbra, and Terumo |
The global endovascular therapy devices market is estimated to be valued at USD 4.8 billion in 2025.
The market size for the endovascular therapy devices market is projected to reach USD 9.3 billion by 2035.
The endovascular therapy devices market is expected to grow at a 6.8% CAGR between 2025 and 2035.
The key product types in endovascular therapy devices market are percutaneous endovascular aneurysm repair (evar), fenestrated evar, aortic stents, biodegradable stents, self-expanding nitinol stents, thoracic aortic aneurysms grafts and other devices.
In terms of procedures, angioplasty with stent placement segment to command 41.2% share in the endovascular therapy devices market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Endovascular Aneurysm Repair (EVAR) Market Size and Share Forecast Outlook 2025 to 2035
Robotic Assisted Endovascular Systems Market Size and Share Forecast Outlook 2025 to 2035
IV Therapy and Vein Access Devices Market Insights – Trends & Forecast 2024-2034
Mesotherapy Market Size and Share Forecast Outlook 2025 to 2035
Cryotherapy Market Growth - Demand, Trends & Emerging Applications 2025 to 2035
Aromatherapy Market Size and Share Forecast Outlook 2025 to 2035
Cell Therapy Systems Market Size and Share Forecast Outlook 2025 to 2035
Chemotherapy-Induced Nausea And Vomiting Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Phototherapy Lamps And Units For Aesthetic Medicine Market Size and Share Forecast Outlook 2025 to 2035
Phototherapy Equipment Market Size and Share Forecast Outlook 2025 to 2035
Phototherapy Treatment Market Size and Share Forecast Outlook 2025 to 2035
Heat Therapy Units Market Analysis - Size, Share, and Forecast 2025 to 2035
Chemotherapy Induced Anemia Market Trends and Forecast 2025 to 2035
Radiotherapy Patient Positioning Accessories Market Trends – Forecast 2025 to 2035
Phototherapy Lamps Market Growth - Trends & Forecast 2025 to 2035
Gene Therapy in CNS Disorder Market Analysis by Indication, Type, End User, and Region through 2035
Radiotherapy Device Market is segmented by External Beam Radiation Therapy Device and Internal Beam Radiation Therapy Device from 2025 to 2035
Chemotherapy-Induced Oral Mucositis Market – Growth & Forecast 2025 to 2035
Chemotherapy-Induced Myelosuppression Treatment Market Growth - Forecast 2025 to 2035
Back Therapy Kit Market Trends – Demand & Future Outlook 2024-2034

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA