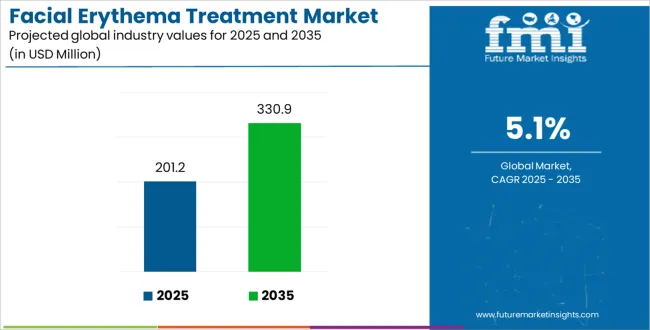
The Facial Erythema Treatment Market is estimated to be valued at USD 201.2 million in 2025 and is projected to reach USD 330.9 million by 2035, registering a compound annual growth rate (CAGR) of 5.1% over the forecast period.
The facial erythema treatment market is experiencing steady expansion, supported by rising awareness of dermatological health, greater access to specialist care, and improved treatment efficacy. Growing patient education about the triggers and symptoms of erythema, combined with higher rates of diagnosis, is driving demand for both prescription and over-the-counter options.
Pharmaceutical companies are investing in new formulations with enhanced anti-inflammatory and vasoconstrictive properties to improve tolerability and long-term outcomes. Regulatory approvals for novel topical therapies and improved reimbursement frameworks are further supporting adoption across diverse demographics.
Additionally, hospital pharmacies and dermatology clinics are playing an increasing role in treatment distribution, reinforcing patient adherence and follow-up care. Looking ahead, the market is expected to benefit from technological advances in teledermatology and digital prescription fulfillment, which are helping to close care gaps and improve disease management.
The market is segmented by Drug Type, Disease Type, Distribution Channel, and Company and region. By Drug Type, the market is divided into Corticosteroids, Emollients, Antihistamines, Antifungal, Antibiotics, and Calcineurin Inhibitors. In terms of Disease Type, the market is classified into Erythematotelangiectatic (ETR), Papulopustular (PPR), Phymatous, and Ocular.
Based on Distribution Channel, the market is segmented into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. By Company, the market is divided into Galderma S.A., Anacor Pharmaceuticals Inc., Astellas Pharma Inc., Meda Pharmaceuticals, Novartis International AG, Pfizer Inc., Regeneron Pharmaceuticals Inc., Sanofi S.A., Valeant Pharmaceuticals International, Inc., and Merck and Co. Inc.
Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
The market is segmented by Drug Type, Disease Type, Distribution Channel, and Company and region. By Drug Type, the market is divided into Corticosteroids, Emollients, Antihistamines, Antifungal, Antibiotics, and Calcineurin Inhibitors. In terms of Disease Type, the market is classified into Erythematotelangiectatic (ETR), Papulopustular (PPR), Phymatous, and Ocular.
Based on Distribution Channel, the market is segmented into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. By Company, the market is divided into Galderma S.A., Anacor Pharmaceuticals Inc., Astellas Pharma Inc., Meda Pharmaceuticals, Novartis International AG, Pfizer Inc., Regeneron Pharmaceuticals Inc., Sanofi S.A., Valeant Pharmaceuticals International, Inc., and Merck and Co. Inc.
Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
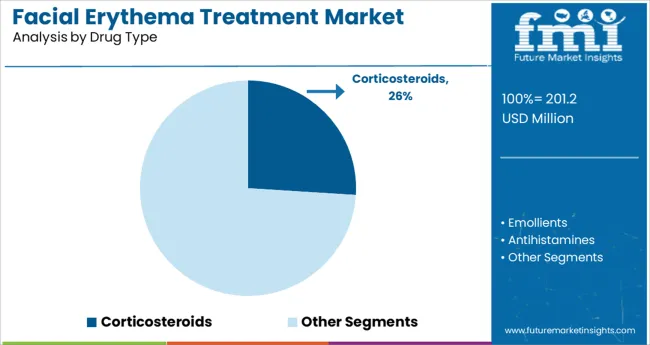
Corticosteroids are projected to account for 26.0% of the total revenue share in the facial erythema treatment market by 2025, making them the leading drug class within prescription therapeutics. This segment’s dominance is being driven by corticosteroids’ proven efficacy in reducing inflammation and suppressing immune-mediated skin responses, which are central to erythema management.
Their broad-spectrum anti-inflammatory activity and established clinical guidelines have made them a first-line therapy for acute flare-ups. Ease of formulation into creams, ointments, and gels further contributes to their widespread use. As patient preference shifts toward treatments offering rapid symptom relief, corticosteroids remain a cornerstone of therapy protocols.
Additionally, continued clinical studies focused on optimizing potency and minimizing long-term side effects are reinforcing confidence in their use across dermatology practices worldwide.
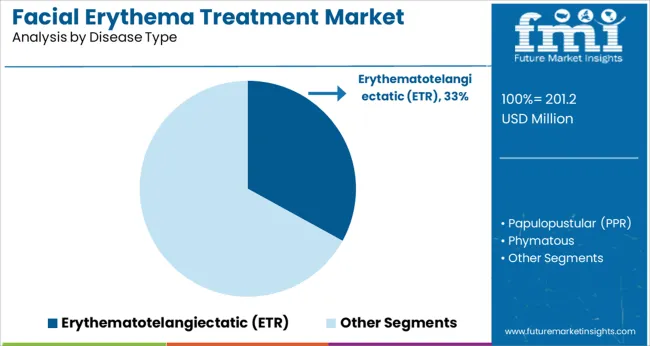
Erythematotelangiectatic (ETR) rosacea is expected to represent 33.0% of the facial erythema treatment market revenue in 2025, making it the most prevalent disease subtype driving therapeutic demand. This segment’s growth is being supported by the high incidence of persistent facial redness and visible blood vessels, which significantly impact patient quality of life and prompt earlier intervention.
Increased awareness campaigns and dermatological screening programs are improving detection rates, while evolving treatment protocols emphasize early pharmacological intervention to prevent progression.
The recurrent nature of ETR and patient concern over cosmetic appearance are motivating consistent treatment adherence and follow-up care. As clinical research uncovers new insights into inflammatory pathways and vascular dysfunction underlying ETR, therapeutic innovation is expected to further strengthen this segment’s position in the market.
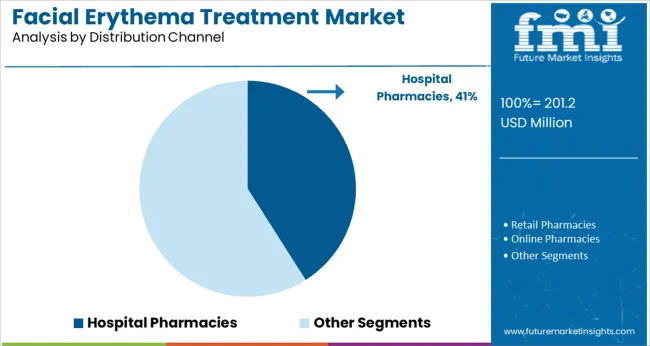
Hospital pharmacies are projected to lead with a 41.0% revenue share of the facial erythema treatment market in 2025. This leadership is being driven by the central role hospitals play in diagnosis, prescription initiation, and dispensing of advanced dermatology therapeutics.
Hospital pharmacies ensure that patients receive counseling on correct usage and monitoring of potential side effects, particularly for corticosteroid and immunomodulatory treatments. Integration with specialist dermatology clinics enables rapid fulfillment of prescriptions and facilitates ongoing patient management.
As complex cases and comorbidities increasingly require coordinated care, hospitals have become preferred channels for dispensing regulated treatments. Additionally, hospital purchasing agreements and formulary inclusion are streamlining access to newer therapies, strengthening this segment’s influence over treatment adoption rates across both urban and regional healthcare settings.
The steadily rising use of alcohol, cigarettes, and other narcotics may result in a variety of skin diseases. Furthermore, changing climatic conditions, the introduction of new products, and allergic reactions all contribute to skin abnormalities such as rosacea, which increases the use of facial erythema treatment.
The demand for minimally invasive procedures is increasing due to advantages such as shorter recovery time and less pain post-procedure. Furthermore, people are becoming increasingly aware of the various types of facial erythema treatment options available on the market. These are important factors that are expected to drive market growth.
People have a growing desire to improve their appearance, especially if they have skin conditions like perioral dermatitis, systemic illness, or rosacea. These, along with the need for skin cancer protection, are expected to significantly drive demand for facial erythema treatment.
Certain medications used during the treatment of facial erythema may cause side effects. Antibiotics, for example, can cause bloating, abdominal pain, loss of appetite, and other side effects. This factor is likely to limit the use of this type of treatment.
In contrast, due to varying utilization demands, key manufacturers are primarily focusing on new product solutions for specific applications. During the forecast period, these factors are expected to generate creative opportunities for market share and increase demand for facial erythema treatment.
North America is the most dominant facial erythema treatment market, accounting for 42% of total revenue. This is due to the majority of the population having fair skin and being prone to skin problems, resulting in the treatment's early adoption.
Climate changes, as well as population lifestyle changes, contribute to a variety of skin diseases. Furthermore, there has been an increase in skin treatment awareness in this region. These factors are likely to sustain the region's growing demand for facial erythema treatment and boost the global market growth.
The US government spends a lot of money on pharmaceuticals and medical devices every year, and they raise awareness in society. Furthermore, the business of the facial erythema treatment market is endorsed by the collaboration of multiple international companies with a slew of major hospital chains.
With revenue of 33.7%, Europe is the second-largest facial erythema treatment market. This is due to rising alcohol consumption and extensive research and development activities in the region.
Most cases of facial erythema, such as rosacea, have no known cause or treatment. It does, however, cause significant discomfort to those who suffer from these skin conditions. In many cases, it causes extreme embarrassment, anxiety, and depression. As a result, start-up companies are taking these issues into account and devising solutions to reduce their prevalence, fueling the growth of the facial erythema treatment market.
Praxis Biotechnology
Dr. Wladis and Dr. Adam founded Praxis Biotechnology in response to a lack of rosacea treatment options. Praxis Biotechnology is based at Albany Medical College. Praxis Biotechnology was recently awarded USD 50,000 after winning FuzeHub's commercialization competition. This medication, according to the non-profit, could benefit millions of people.
Legit Health
Legit Health is a Spanish start-up that offers a skin diagnostic tool. The startup's software employs artificial intelligence to classify 139 skin pathologies, with a focus on the detection of seven of these, including acne, rosacea, and dermatitis. The software's algorithms automatically fill in most medical grading systems for the seven skin conditions.
Dermatica
The team at this UK start-up develops an evidence-based treatment and delivers it monthly. The web-based platform of the start-up assists patients in developing relationships with medical professionals and seeking expert assistance when necessary. The company also develops treatments for acne, facial erythema, anti-aging, pigmentation, and melasma.
Due to the involvement of several established players, the market share for facial erythema treatment is highly competitive. These players are focusing on expanding their presence through acquisitions, expansions, product approvals, and launches in order to capitalize on market growth opportunities.
Latest Developments:
Other Market Participants of the Facial Erythema Treatment Market:
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 5.1% from 2025 to 2035 |
| The base year for estimation | 2024 |
| Historical data | 2020 to 2024 |
| Forecast period | 2025 to 2035 |
| Quantitative units | Revenue in USD million, volume in kilotons, and CAGR from 2025 to 2035 |
| Report Coverage | Revenue forecast, volume forecast, company ranking, competitive landscape, growth factors, and trends, Pricing Analysis, |
| Segments Covered | Drug Type, Disease Type, Distribution Channel, and Region. |
| Regional scope | North America; Western Europe; Eastern Europe; Middle East; Africa, ASEAN; South Asia; Rest of Asia; Australia; and New Zealand |
| Country scope | United States of America, Canada; Mexico, Germany, United Kingdom, France, Italy, Spain, Russia, Belgium, Poland, Czech Republic, China, India, Japan, Australia, Brazil, Argentina, Colombia, Saudi Arabia, UAE, Iran, South Africa |
| Key companies profiled | Anacor Pharmaceuticals Inc.; Astellas Pharma Inc.; Galderma S.A.; Meda Pharmaceuticals; Pfizer Inc.; Novartis International AG; Regeneron Pharmaceuticals Inc.; Sanofi S.A.; Valeant Pharmaceuticals International Inc.; Bausch Health Companies Inc.; GlaxoSmithKline plc; Merck & Co. Inc.; Enzon Pharmaceuticals; etc. |
| Customization scope | Free report customization (equivalent to up to 8 analysts' working days) with purchase. Addition or alteration to country, regional & segment scope. |
| Pricing and purchase options | Avail customized purchase options to meet your exact research needs. |
The global facial erythema treatment market is estimated to be valued at USD 201.2 million in 2025.
It is projected to reach USD 330.9 million by 2035.
The market is expected to grow at a 5.1% CAGR between 2025 and 2035.
The key product types are corticosteroids, emollients, antihistamines, antifungal, antibiotics and calcineurin inhibitors.
erythematotelangiectatic (etr) segment is expected to dominate with a 33.0% industry share in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Facial Serum Market Forecast and Outlook 2025 to 2035
Facial Care Products Market Size and Share Forecast Outlook 2025 to 2035
Facial Recognition Market Size and Share Forecast Outlook 2025 to 2035
Facial Mask Maker Market Size and Share Forecast Outlook 2025 to 2035
Facial Oil Market Analysis by Product Type, End User, Sales Channel and Region Through 2025 to 2035
Facial Tissue Paper Market Analysis - Trends, Growth & Forecast 2025 to 2035
Facial Steamer Market - Trends, Growth & Forecast 2025 to 2035
Facial Pumps Market Growth – Demand & Forecast 2025 to 2035
Facial Tracking Solution Market by Component, Technology Type, Industry & Region Forecast till 2035
Facial Tissue Market Growth – Size, Demand & Forecast 2024-2034
Facial Epilator Market Analysis – Size, Trends & Forecast 2024-2034
Facial Implants Market
Facial Recognition Machine Market
Bifacial Solar Module Market Size and Share Forecast Outlook 2025 to 2035
Craniofacial Implants Market Size and Share Forecast Outlook 2025 to 2035
Electric Facial Cleansing Brush Market Size and Share Forecast Outlook 2025 to 2035
Chelating Facial Masks Market Analysis - Size and Share Forecast Outlook 2025 to 2035
Gluten-Free Facial Product Market Trends – Growth & Forecast 2024-2034
Cranial And Facial Implants Market
Craniomaxillofacial Devices Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA