The meningococcal meningitis treatment market is valued at USD 195.98 million in 2025. As per FMI analysis, the industry will grow at a CAGR of 4.7% and reach USD 310.23 million by 2035.
In 2024, the industry experienced steady growth due to increased awareness campaigns and expanded vaccination programs across emerging economies. Governments and non-profit organizations collaborated to provide affordable vaccines in high-risk regions, notably in the African meningitis belt. Enhanced diagnostic capabilities, including rapid antigen detection tests, improved early diagnosis rates, further contributing to industry growth.
Pharmaceutical companies focused on research and development, leading to the introduction of more effective conjugate vaccines and antibiotic treatments. Additionally, regulatory approvals for new combination vaccines accelerated product adoption.
In 2025 and beyond, the industry is expected to witness further expansion as governments strengthen immunization policies and increase healthcare investments. Pharmaceutical innovations, including next-generation vaccines with broader serogroup coverage, will enhance industry competitiveness. Public-private partnerships will continue to improve accessibility to treatments in underserved areas.
Furthermore, technological advancements in diagnostic tools will support early detection and management. With increasing global travel and urbanization, the demand for preventive vaccinations will rise, contributing to sustained industry growth. Manufacturers are likely to focus on strategic collaborations to expand their geographical presence and strengthen distribution networks.
Meningococcal Meningitis Treatment Market Statistics
| Metric | Value |
|---|---|
| Industry Size (2025E ) | USD 195.98 million |
| Industry Value (2035F) | USD 310.23 million |
| CAGR (2025 to 2035) | 4.7% |
The industry is set for steady growth, driven by increased vaccination initiatives, rising disease awareness, and advancements in diagnostic technologies. Pharmaceutical companies and vaccine manufacturers will benefit from expanding industry opportunities, particularly in emerging economies with high disease prevalence. However, regions with limited healthcare infrastructure may struggle to keep pace, posing challenges for equitable treatment access.
Expand Vaccination Accessibility
Invest in partnerships with governments and NGOs to enhance vaccination programs, especially in high-risk regions. Prioritize affordable vaccine distribution and strengthen cold chain logistics.
Innovate in Product Development
Focus on R&D to create next-generation vaccines with broader serogroup coverage and longer-lasting immunity. Adapt to evolving regulatory standards and accelerate product approvals.
Strengthen Industry Presence
Establish strategic alliances and expand distribution networks in emerging industries. Explore M&A opportunities to gain regional industry share and diversify product portfolios.
| Risk | Details |
|---|---|
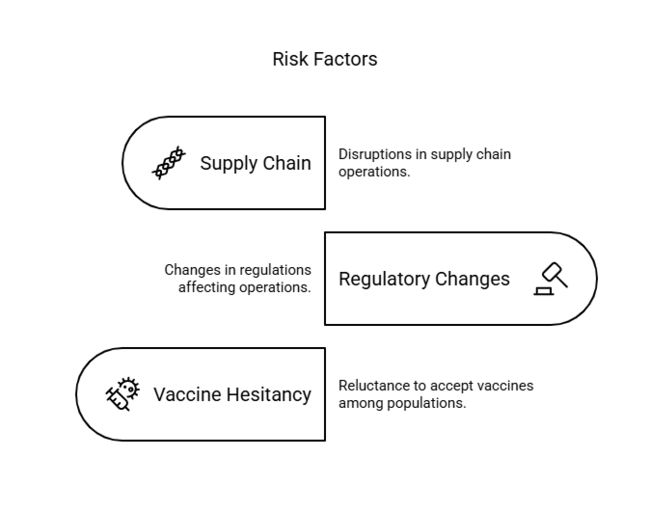
| Supply Chain Disruptions | Probability: High, Impact: High |
| Regulatory Changes | Probability: Medium, Impact: High |
| Vaccine Hesitancy | Probability: Medium, Impact: Medium |
| Priority | Immediate Action |
|---|---|
| Expand Supply Chain Resilience | Evaluate alternative suppliers and establish backup logistics channels. |
| Monitor Regulatory Environment | Stay engaged with policymakers and ensure compliance with evolving regulations. |
| Boost Vaccine Awareness | Partner with healthcare organizations to launch targeted educational campaigns. |
To stay ahead, companies must proactively strengthen supply chain resilience by diversifying suppliers and building redundancy in critical regions. Investing in innovative vaccine development with broader serogroup coverage will be key to gaining a competitive edge. Additionally, forming strategic partnerships with governments and NGOs can enhance industry access in underserved areas.
Companies should also closely monitor regulatory landscapes and engage with policymakers to stay compliant and influence favorable outcomes. Lastly, targeted awareness campaigns and stakeholder collaboration will be essential to combat vaccine hesitancy and drive industry demand.
| Country | Policies & Regulations Impacting the Industry |
|---|---|
| USA | FDA regulations for vaccine approval and manufacturing; CDC's Vaccination Schedule mandates meningococcal vaccines for adolescents and high-risk groups. |
| UK | NHS offers free meningococcal vaccines through national immunization programs; MHRA regulates drug approvals. |
| France | Mandatory vaccination for infants under the National Immunization Plan; ANSM ensures vaccine safety and efficacy. |
| Germany | Stiko (Standing Committee on Vaccination) recommends meningococcal vaccines; PEI (Paul-Ehrlich-Institut) manages regulatory compliance. |
| Italy | The National Vaccine Prevention Plan mandates vaccinations for adolescents, and the AIFA oversees vaccine quality and supply chain compliance. |
| South Korea | KCDC recommends meningococcal vaccines for military personnel and high-risk individuals; MFDS controls vaccine safety and efficacy. |
| Japan | Vaccination is not mandatory; PMDA regulates product approvals, focusing on safety trials before industry entry. |
| China | Meningococcal vaccines are included in the National Immunization Program for Children; NMPA ensures strict vaccine testing and quality standards. |
| Australia-NZ | Government-funded vaccination programs for adolescents, TGA (Australia) and Medsafe (NZ) regulate vaccine approvals and monitor safety. |
The USA is anticipated to grow with a CAGR of 4.5% between 2025 and 2035. Strong government support for vaccination programs, along with strong R&D investments and sophisticated healthcare infrastructure, are some of the factors that have contributed to driving the industry. The presence of large pharmaceutical companies and favorable regulatory policies also support the industry's growth. There have been advancements in technology and the development of new medications. Additionally, growing public awareness about health improvement has led to an increased demand for vaccines, particularly for emerging infectious diseases. For sustained industry growth, government agencies and private sector players work collaboratively.
The UK is expected to grow by 4.3% from 2025 to 2035. Government focus on public health, along with the rise in immunization programs, isa major factor in driving the growth of the industry. The country’s efficient regulatory processes and inroads in combating infectious diseases offer a friendly environment for vaccine manufacturers. It also enables innovation through partnerships with academic institutions and biopharma companies. The rise in investments in healthcare infrastructure and technological innovations further contributes to the expansion of the industry.
France is expected to grow by 4.1% between 2025 and 2035. Preventive healthcare is prioritized in the country with proactive government immunization campaigns in place driving growth in the industry. France has a well-established pharmaceutical sector, combined with significant R&D investments, supports the development and distribution of vaccines. Similarly, the need for public awareness campaigns and school-based vaccination programs are important growth factors in the industry. Additionally, the industry growth is supported by the government's emphasis on controlling infectious diseases and sustaining high rates of immunization coverage.
Germany is anticipated to grow at a CAGR of 4.6% from 2025 to 2035. Germany is another strong player in the vaccine industry, having a solid pharmaceutical industry and a high investment in biotechnology. Furthermore, government measures to promote vaccine development and production, along with a strong healthcare infrastructure sustain industry growth. Collaborations between public and private institutions also stimulate innovation. Additionally, the focus on advanced vaccines and immunization coverage is expected to strengthen the industry's growth in the coming years.
Italy is anticipated to grow with a CAGR of 4.0% in the period 2025 to 2035. Industry growth is motivated by the concentration of vaccination programs in the country's healthcare system and the government's support for immunization campaigns. Innovative vaccines are produced through encouraging partnerships between pharmaceutical firms and research organizations. Steady industry growth of vaccine-preventable diseases is attributed to growing public awareness of invasive therapeutic areas. Additionally, the implementation of national immunization plans has further supported this growth.
South Korea is anticipated to grow at a CAGR of 4.8%. Strong government initiatives and public-private partnerships in the biotechnology sector promote the vaccine industry. Significant investments in R&D are aimed at advanced vaccine development, further driving industry growth. The growing number of vaccine-preventable diseases has increased the demand for vaccines. Additionally, a well-established healthcare infrastructure and proactive immunization campaigns further drive this demand. South Korea's effort at combating infectious diseases allows the industry to grow.
Japan is anticipated to grow at a CAGR of 4.2% during the period of 2025 to 2035. The country's healthcare policies focus on preventive care and early immunization, fuelling industry growth. In addition, government support of vaccine research and collaboration between academics and industry contribute to innovation. Japan's aging demographic also drives demand for vaccines targeting age-related diseases. The growth of the industry is propelled by strong public awareness and effective healthcare infrastructure.
China is anticipated to grow at a CAGR of 5.1% from 2025 to 2035. The country’s large population and increasing government investments in healthcare infrastructure drive industry growth. China’s commitment to expanding immunization coverage and combating infectious diseases supports the vaccine industry’s expansion. Additionally, the country’s rapidly growing biotechnology sector and emphasis on domestic vaccine production enhance industry competitiveness. Collaborations with international organizations further strengthen China’s position in the global vaccine industry.
Australia and New Zealand are anticipated to grow at a CAGR of 4.4%.Industry growth is driven by the countries’ strong focus on public health and comprehensive immunization programs. Industry growth is also aided by government initiatives to make the vaccine accessible and affordable to the population.
It also spurs more innovationthrough collaborations between research institutions and pharmaceutical companies. The industrywitnessed growth in the country, backed by the dedication of the government to achieving vaccination campaigns and public health awareness programs in the region.
The meningococcal meningitis treatment industry is segmented by causative micro-organisms into bacterial, viral, and fungal. By treatment type, it includes antibiotic therapy-penicillin, ampicillin, chloramphenicol, and ceftriaxone-and adjunctive therapy. The route of administration is comprised of injectable and oral. Vaccine types include meningococcal conjugate vaccine, meningococcal polysaccharide vaccine, and combination vaccine. Distribution channels are hospital pharmacies, retail pharmacies, and online pharmacies.
Vaccine Type remains the leading segment, with a projected CAGR of 5.0% from 2025 to 2035. The dominance of this segment is driven by the continuous development of advanced vaccines targeting both infectious diseases and non-communicable conditions. Innovations in mRNA vaccines and recombinant vaccines are fueling growth, along with increased government investments in vaccine research and production. Additionally, the rising prevalence of chronic diseases and the emphasis on preventative healthcare through vaccination programs are expected to sustain the robust performance of this segment.
The Causative Micro-organism segment is anticipated to grow at a CAGR of 4.9%. The heightened focus on understanding the genetic and structural composition of pathogens has accelerated vaccine development efforts. Emerging and re-emerging infectious diseases, such as influenza, respiratory syncytial virus (RSV), and new COVID-19 variants, are driving demand. Additionally, advancements in genomic studies and molecular biology have improved the accuracy and efficacy of vaccines, contributing to the rapid growth of this segment.
The Route of Administration segment is expected to witness a CAGR of4.8%. Injectable vaccines remain the most common form of administration, particularly for large-scale immunization programs. However, intranasal, oral, and transdermal vaccine delivery systems are gaining popularity due to their convenience, non-invasive nature, and ease of use. Ongoing research into needle-free and self-administrable vaccine technologies is further propelling growth in this segment.
The Distribution Channel segment is projected to grow at a CAGR of 4.6%. While hospitals and government vaccination programs continue to dominate vaccine distribution, pharmacies and retail healthcare providers are becoming increasingly prominent. Expanding vaccine access through partnerships with pharmacy chains and e-pharmacies ensures greater reach in both urban and rural areas. The emphasis on efficient cold chain management systems also supports the growth of this segment.
The Treatment Type segment is estimated to grow at a CAGR of 4.5%. Preventive vaccines remain the cornerstone of the segment, especially for infectious diseases. However, therapeutic vaccines, particularly for cancer and autoimmune disorders, are gaining traction. Continuous research in personalized vaccine therapies and advancements in immunotherapy are contributing to the steady growth of this segment. Additionally, increased funding for vaccine R&D ensures a consistent pipeline of new treatments, further supporting industry expansion.
In this industry, key global pharmaceutical players are strategically aligning their portfolios to address evolving public health priorities and immunization strategies. Leading manufacturers such as GlaxoSmithKline (GSK), Merck & Co., Sanofi, Pfizer, and Novavax are driving advancements through expanded vaccine development and global distribution agreements.
These companies are not only investing in R&D for enhanced conjugate and combination vaccines but are also leveraging international collaborations to strengthen their industry presence. For instance, Sanofi and Novavax recently announced a strategic alliance to co-develop and commercialize combination vaccines targeting respiratory diseases, including COVID-19 and influenza, which indirectly supports innovation capacity in the meningitis space.
Distributors and regional suppliers are capitalizing on public sector tenders and global health initiatives by organizations such as Gavi and WHO to enhance vaccine access in endemic regions. Meanwhile, manufacturers like GSK are adjusting commercial strategies due to fluctuating demand forecasts, as seen with their revised outlook for RSV vaccine sales in 2024.
Amid shifting vaccine priorities post-pandemic, players such as Moderna have faced revenue constraints due to waning COVID-19 vaccine demand, raising concerns about pipeline sustainability. Nevertheless, the focus on the lifecycle management of meningitis vaccines and antibiotic therapies remains strong. Industry stakeholders are emphasizing cold-chain distribution, improved healthcare access, and integrated treatment-vaccine protocols to ensure continued protection against meningococcal meningitis through 2035.
In 2025, the space will be dominated by a few key pharmaceutical players who have consolidated their positions through strategic R&D, global partnerships, and vaccine deployment in high-burden regions. Pfizer retains its leadership with an estimated 24% market share, supported by regulatory advancements for its MenABCWY vaccine and expanded procurement agreements in Sub-Saharan Africa.
GlaxoSmithKline (GSK) strengthens its share to 19% after scaling Menveo production under Gavi-supported campaigns. Sanofi remains influential at 17%, driven by ongoing trials and partnerships with biotech firms for next-gen MenB vaccines. Merck & Co. sustains its relevance at 13%, leveraging technology platforms and CDC-preferred vaccines. Novartis, having exited direct vaccine manufacturing, maintains a residual 9% share from legacy distribution and royalties.
| Company | Estimated Market Share (2025) |
|---|---|
| Pfizer | 24% |
| GlaxoSmithKline (GSK) | 19% |
| Sanofi | 17% |
| Merck & Co. | 13% |
| Novartis | 9% |
The industry is segmented into bacterial, viral, fungal.
The industry is segmented into antibiotic Therapy.
The industry is bifurcated into injectable and oral.
The industry is divided into meningococcal conjugate vaccine, meningococcal polysaccharide vaccine, and combination vaccine.
The industry is segmented into hospital pharmacy, retail pharmacy, and online pharmacy.
The industry is segmented intoNorth America, Latin America, Europe, South Asia, East Asia, Oceania, and Middle East & Africa.
Antibiotics like ceftriaxone and cefotaxime are commonly prescribed as they effectively target Neisseria meningitidis, the bacteria responsible for the infection.
Combination therapies involving antibiotics and supportive care, including corticosteroids to reduce inflammation, are becoming more widely adopted for better patient outcomes.
Telemedicine platforms facilitate early diagnosis and remote monitoring, especially in regions with limited healthcare access, ensuring timely treatment.
North America and Europe prioritize advanced antibiotic therapies and vaccinations, while low-income regions often rely on reactive treatment during outbreaks.
Monoclonal antibodies and next-generation vaccines are emerging as promising options, aiming to provide long-term immunity and reduce disease severity.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 5: Global Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 6: Global Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 9: North America Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 10: North America Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 11: North America Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 12: North America Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Table 13: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Latin America Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 15: Latin America Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 16: Latin America Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 17: Latin America Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 18: Latin America Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Table 19: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 20: Europe Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 21: Europe Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 22: Europe Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 23: Europe Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 24: Europe Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Table 25: South Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 26: South Asia Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 27: South Asia Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 28: South Asia Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 29: South Asia Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 30: South Asia Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Table 31: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 32: East Asia Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 33: East Asia Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 34: East Asia Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 35: East Asia Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 36: East Asia Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Table 37: Oceania Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 38: Oceania Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 39: Oceania Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 40: Oceania Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 41: Oceania Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 42: Oceania Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Table 43: MEA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 44: MEA Market Value (US$ Million) Forecast by Causative Micro-organism, 2018 to 2033
Table 45: MEA Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 46: MEA Market Value (US$ Million) Forecast by Route of Administration , 2018 to 2033
Table 47: MEA Market Value (US$ Million) Forecast by Vaccine Type, 2018 to 2033
Table 48: MEA Market Value (US$ Million) Forecast by Distribution channel, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 4: Global Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 5: Global Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 6: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 7: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 8: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 9: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 10: Global Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 11: Global Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 12: Global Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 13: Global Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 14: Global Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 15: Global Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 16: Global Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 17: Global Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 18: Global Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 19: Global Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 20: Global Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 21: Global Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 22: Global Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 23: Global Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 24: Global Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 25: Global Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 26: Global Market Attractiveness by Treatment Type, 2023 to 2033
Figure 27: Global Market Attractiveness by Route of Administration , 2023 to 2033
Figure 28: Global Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 29: Global Market Attractiveness by Distribution channel, 2023 to 2033
Figure 30: Global Market Attractiveness by Region, 2023 to 2033
Figure 31: North America Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 32: North America Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 33: North America Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 34: North America Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 35: North America Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 36: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 37: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 38: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 39: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 40: North America Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 41: North America Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 42: North America Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 43: North America Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 44: North America Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 45: North America Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 46: North America Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 47: North America Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 48: North America Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 49: North America Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 50: North America Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 51: North America Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 52: North America Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 53: North America Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 54: North America Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 55: North America Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 56: North America Market Attractiveness by Treatment Type, 2023 to 2033
Figure 57: North America Market Attractiveness by Route of Administration , 2023 to 2033
Figure 58: North America Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 59: North America Market Attractiveness by Distribution channel, 2023 to 2033
Figure 60: North America Market Attractiveness by Country, 2023 to 2033
Figure 61: Latin America Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 62: Latin America Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 63: Latin America Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 64: Latin America Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 65: Latin America Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 66: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 67: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 68: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 69: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 70: Latin America Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 71: Latin America Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 72: Latin America Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 73: Latin America Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 74: Latin America Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 75: Latin America Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 76: Latin America Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 77: Latin America Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 78: Latin America Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 79: Latin America Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 80: Latin America Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 81: Latin America Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 82: Latin America Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 83: Latin America Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 84: Latin America Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 85: Latin America Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 86: Latin America Market Attractiveness by Treatment Type, 2023 to 2033
Figure 87: Latin America Market Attractiveness by Route of Administration , 2023 to 2033
Figure 88: Latin America Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 89: Latin America Market Attractiveness by Distribution channel, 2023 to 2033
Figure 90: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 91: Europe Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 92: Europe Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 93: Europe Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 94: Europe Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 95: Europe Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 96: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 97: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 98: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 99: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 100: Europe Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 101: Europe Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 102: Europe Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 103: Europe Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 104: Europe Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 105: Europe Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 106: Europe Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 107: Europe Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 108: Europe Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 109: Europe Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 110: Europe Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 111: Europe Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 112: Europe Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 113: Europe Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 114: Europe Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 115: Europe Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 116: Europe Market Attractiveness by Treatment Type, 2023 to 2033
Figure 117: Europe Market Attractiveness by Route of Administration , 2023 to 2033
Figure 118: Europe Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 119: Europe Market Attractiveness by Distribution channel, 2023 to 2033
Figure 120: Europe Market Attractiveness by Country, 2023 to 2033
Figure 121: South Asia Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 122: South Asia Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 123: South Asia Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 124: South Asia Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 125: South Asia Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 126: South Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 127: South Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 128: South Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 129: South Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 130: South Asia Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 131: South Asia Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 132: South Asia Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 133: South Asia Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 134: South Asia Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 135: South Asia Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 136: South Asia Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 137: South Asia Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 138: South Asia Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 139: South Asia Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 140: South Asia Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 141: South Asia Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 142: South Asia Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 143: South Asia Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 144: South Asia Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 145: South Asia Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 146: South Asia Market Attractiveness by Treatment Type, 2023 to 2033
Figure 147: South Asia Market Attractiveness by Route of Administration , 2023 to 2033
Figure 148: South Asia Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 149: South Asia Market Attractiveness by Distribution channel, 2023 to 2033
Figure 150: South Asia Market Attractiveness by Country, 2023 to 2033
Figure 151: East Asia Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 152: East Asia Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 153: East Asia Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 154: East Asia Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 155: East Asia Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 156: East Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 157: East Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 158: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 159: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 160: East Asia Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 161: East Asia Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 162: East Asia Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 163: East Asia Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 164: East Asia Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 165: East Asia Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 166: East Asia Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 167: East Asia Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 168: East Asia Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 169: East Asia Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 170: East Asia Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 171: East Asia Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 172: East Asia Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 173: East Asia Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 174: East Asia Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 175: East Asia Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 176: East Asia Market Attractiveness by Treatment Type, 2023 to 2033
Figure 177: East Asia Market Attractiveness by Route of Administration , 2023 to 2033
Figure 178: East Asia Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 179: East Asia Market Attractiveness by Distribution channel, 2023 to 2033
Figure 180: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 181: Oceania Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 182: Oceania Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 183: Oceania Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 184: Oceania Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 185: Oceania Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 186: Oceania Market Value (US$ Million) by Country, 2023 to 2033
Figure 187: Oceania Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 188: Oceania Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 189: Oceania Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 190: Oceania Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 191: Oceania Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 192: Oceania Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 193: Oceania Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 194: Oceania Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 195: Oceania Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 196: Oceania Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 197: Oceania Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 198: Oceania Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 199: Oceania Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 200: Oceania Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 201: Oceania Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 202: Oceania Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 203: Oceania Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 204: Oceania Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 205: Oceania Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 206: Oceania Market Attractiveness by Treatment Type, 2023 to 2033
Figure 207: Oceania Market Attractiveness by Route of Administration , 2023 to 2033
Figure 208: Oceania Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 209: Oceania Market Attractiveness by Distribution channel, 2023 to 2033
Figure 210: Oceania Market Attractiveness by Country, 2023 to 2033
Figure 211: MEA Market Value (US$ Million) by Causative Micro-organism, 2023 to 2033
Figure 212: MEA Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 213: MEA Market Value (US$ Million) by Route of Administration , 2023 to 2033
Figure 214: MEA Market Value (US$ Million) by Vaccine Type, 2023 to 2033
Figure 215: MEA Market Value (US$ Million) by Distribution channel, 2023 to 2033
Figure 216: MEA Market Value (US$ Million) by Country, 2023 to 2033
Figure 217: MEA Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 218: MEA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 219: MEA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 220: MEA Market Value (US$ Million) Analysis by Causative Micro-organism, 2018 to 2033
Figure 221: MEA Market Value Share (%) and BPS Analysis by Causative Micro-organism, 2023 to 2033
Figure 222: MEA Market Y-o-Y Growth (%) Projections by Causative Micro-organism, 2023 to 2033
Figure 223: MEA Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 224: MEA Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 225: MEA Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 226: MEA Market Value (US$ Million) Analysis by Route of Administration , 2018 to 2033
Figure 227: MEA Market Value Share (%) and BPS Analysis by Route of Administration , 2023 to 2033
Figure 228: MEA Market Y-o-Y Growth (%) Projections by Route of Administration , 2023 to 2033
Figure 229: MEA Market Value (US$ Million) Analysis by Vaccine Type, 2018 to 2033
Figure 230: MEA Market Value Share (%) and BPS Analysis by Vaccine Type, 2023 to 2033
Figure 231: MEA Market Y-o-Y Growth (%) Projections by Vaccine Type, 2023 to 2033
Figure 232: MEA Market Value (US$ Million) Analysis by Distribution channel, 2018 to 2033
Figure 233: MEA Market Value Share (%) and BPS Analysis by Distribution channel, 2023 to 2033
Figure 234: MEA Market Y-o-Y Growth (%) Projections by Distribution channel, 2023 to 2033
Figure 235: MEA Market Attractiveness by Causative Micro-organism, 2023 to 2033
Figure 236: MEA Market Attractiveness by Treatment Type, 2023 to 2033
Figure 237: MEA Market Attractiveness by Route of Administration , 2023 to 2033
Figure 238: MEA Market Attractiveness by Vaccine Type, 2023 to 2033
Figure 239: MEA Market Attractiveness by Distribution channel, 2023 to 2033
Figure 240: MEA Market Attractiveness by Country, 2023 to 2033






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Meningitis Treatment Market Analysis - Growth & Forecast 2025 to 2035
Meningococcal Disease Treatment Market
Tuberculous Meningitis Treatment Market - Demand & Innovations 2025 to 2035
Meningococcal Vaccine Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market
Water Treatment System Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Chemical Market Size and Share Forecast Outlook 2025 to 2035
Algae Treatment Chemical Market Forecast and Outlook 2025 to 2035
Water Treatment Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Equipment Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA