Global monkeypox treatment market is anticipated to grow at a robust CAGR over the forecast period, 2025 to 2035, owing to the rising prevalence of monkeypox outbreaks across the globe and increasing need for effective antiviral treatments and vaccines.
Government initiatives, research funding, and collaborations between pharmaceutical companies are speeding drug development and treatment strategies. A rising awareness of zoonotic diseases and their potential to cause infections such as the current outbreak is also helping the market.
This market is further projected to grow, due to the growing demand for rapid diagnostic solutions, supportive care treatments and antiviral drugs. But thanks to continued medical research, the pipeline of new therapies is long: there are more and more options for how we can care for patients and deal with outbreaks. : Focus on strengthening healthcare infrastructure, global preparedness for migrant outbreaks, and access to vaccines will be major market drivers in the upcoming years.
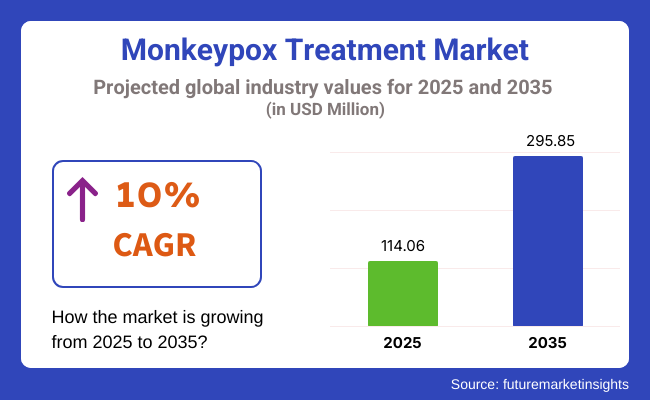
In 2025, the monkeypox treatment market was valued at approximately USD 114.06 million. By 2035, it is projected to reach USD 295.85 million, reflecting a compound annual growth rate (CAGR) of 10%. Market expansion is driven by increased government funding for infectious disease research, advancements in virology, and the introduction of novel antiviral treatments.
Additionally, rising investments in biopharmaceuticals and vaccine development are expected to fuel market growth. The integration of digital health solutions for real-time disease tracking, telemedicine for patient care, and AI-driven drug discovery is further supporting the market.
Moreover, the focus on developing non-invasive treatment methods, combination therapies, and post-exposure prophylaxis is expanding therapeutic options. Companies are investing in supply chain resilience, production scalability, and equitable vaccine distribution to address future outbreaks efficiently.
North America is the leading region in the global monkeypox treatment market owing to adequate awareness, advanced research capabilities, and well-structured healthcare infrastructure in the region. The United States and Canada are pouring money into vaccine production, stockpiling antiviral drugs and ramping up public health emergency responses.
Key players within the region that focuses on clinical trials, speed regulatory approvals, and distribution channels for an effective disease management. Also, the CDC and FDA are government agencies that also fund research and administer the vaccines.
Massive partnerships between biotech firms, academic institutions and government agencies are enabling rapid development of drugs and vaccines. Telemedicine, digital disease tracking, and AI-driven diagnostics all of these innovations are quickly being adopted as best practices for outbreak prevention and response in North America.
Europe's market is described by increasing research partnerships, robust regulatory frameworks and large investments in infectious disease prevention. Countries like Germany, the UK, France and Italy lead vaccine development, antiviral therapies and public health measures.
The EMA is fast-tracking monkeypox treatment approvals to facilitate access to medical solutions. Increasing government support and collaboration between pharmaceutical companies is adding power to research funding and production capacity. Greater attention to genetic sequencing, epidemiological surveillance and early-intervention strategies is only boosting Europe’s muscle to battle outbreaks more broadly.
The rapid growth of the market is also being ensured with the advancements being made in the manufacturing capacity of biopharmaceuticals along with the improvements being made in the supply chain process.
With increased healthcare investments, heightened awareness of zoonotic diseases, and growing vaccine manufacturing capabilities, the Asia-Pacific region is anticipated to grow at the fastest rate. Research on antivirals and immune-response therapies is being prioritized as part of public health security in countries such as China, Japan, South Korea, and India.
Regional market growth is driven by government initiatives such as improving vaccine distribution capabilities and diagnostic capabilities, and establishing strategic partnerships. Furthermore, partnerships with global organizations including WHO and Gavi are promoting equitable access to treatments for other world countries.
The growing shift towards novel biopharmaceutical technologies, such as mRNA vaccine platforms and artificial intelligence (AI)-driven drug discovery, is driving the market forward. And with expanding infrastructure around clinical trials and greater research funding, new treatment options are accelerating in the region.
The monkeypox treatment market will witness considerable growth in the next decade owing to the continuous investment in research and development, collaborations across the globe, and proceeding technological advancements.
The future of this market will be driven by the development of AI-powered diagnostics, new antiviral drugs, and optimized vaccine distribution strategies. Government spending on public health programs, expanding biopharmaceutical production, and strengthening global collaboration will boost the market and widen the access to efficacious medicines across nations.
Challenge
Stringent Regulatory Compliance and Standards
Monkeypox treatment market to showcase lavish growth in the consecutive years gauge over the global monkeypox treatment market due to stringent regulation across the globe, making treatment accounting or sterilization and disinfection equipment.
A number of agencies, such as the FDA, EU MDR, and ISO ensure patient safety through stringent guidelines, forcing manufacturers to comply with high standards of efficacy and safety. Staying ahead of these emerging rules requires continual spending on research, testing and certification processes.
In order to traverse this maze of regulatory requirements, organizations need to be abreast with global standards and regulations, invest in advanced compliance solutions and pursue effective quality control interventions.
High Costs and Limited Adoption in Developing Regions
Medical cleaning devices typically entail a high investment due to the requirement for advanced technology, automated sterilization systems, and sustainable disinfection solutions. Enormous initial investments and maintenance costs are deterrents, especially for medical facilities in developing parts of the world with limited budgets.
Also, widespread adoption is hindered by a lack of awareness and infrastructure the issue here is that companies need to look at affordable manufacturing, financing options, and do awareness programs for further market penetration in areas where there is no adequate coverage.
Opportunity
Rising Demand for Infection Control in Healthcare Settings
The increasing focus on infection prevention and patient safety is propelling demand for advanced medical cleaning devices. Among them, Hospital-acquired infections (HAIs) pose a critical challenge; consequently, healthcare places invest in efficient sterilization and disinfection solutions.
Such automation trends, as well as the growing penetration of automation technologies like automated cleaning systems, UV-C disinfection technology, and AI-based sterilization monitoring, are including ample opportunities for the expansion of the sterilization market. Such advancements will lead to increased competition, thus driving companies to develop innovative, intuitive, and high-performance cleaning appliances.
Technological Advancements in Sterilization and Disinfection
Additionally, ultrasonic cleaning, hydrogen peroxide vapor sterilization, and robotic disinfection systems are similar in that they represent advances in cleaning technology that are changing the face of the medical cleaning market and making the cleaning process more efficient and effective.
From AI-powered monitoring systems to smart sterilization tracking through the Internet of Things (IoT) and eco-friendly cleaning agents, innovative solutions are emerging that enable healthcare facilities to take the reins of infection prevention. Company’s spending money on conventional investing in research and development, sustainability and automation will capitalize on the demand for next-generation medical cleaning devices.
Due to increased infection control measures following the COVID-19 pandemic, monkeypox treatmentmarket registered robustmarket growth between2020 through 2024. The rise in demand for automated sterilization, eco-friendly disinfectants, and AI-integrated monitoring systems as hospitals and healthcare facilities prioritize hygiene drove this growth.
Although challenges like high costs and supply chain disruptions posed barriers to market expansion. In response, companies invested in R&D, improved distribution networks, and rolled out affordable solutions with emerging markets in mind.
The future trends till 2025 to 2035 will focus on smart and sustainable sterilization technologies. Automation in the form of AI-driven analytics, robotic cleaning systems, and biodegradable disinfection solutions will be mainstreaming the market.
Regulatory guidelines will become more stringent for sterilization, compelling manufacturers to move toward state-of-the-art, sustainable manufacturing processes. The next decade will be dominated by companies engaged in automation, sustainability and digital transformation.
Market Shifts: A Comparative Analysis 2020 to 2024 vs. 2025 to 2035
| Market Shift | 2020 to 2024 Trends |
|---|---|
| Regulatory Landscape | Stricter post-pandemic hygiene regulations |
| Technological Advancements | Growth in UV-C and ultrasonic sterilization |
| Industry Adoption | Increased use in hospitals and clinics |
| Supply Chain and Sourcing | Dependence on traditional sterilization suppliers |
| Market Competition | Dominance of established medical equipment manufacturers |
| Market Growth Drivers | Increased hospital infection control spending |
| Sustainability and Energy Efficiency | Initial focus on reducing chemical waste |
| Integration of Smart Monitoring | Limited adoption of digital tracking |
| Advancements in Product Innovation | Traditional chemical-based disinfection solutions |
| Market Shift | 2025 to 2035 Projections |
|---|---|
| Regulatory Landscape | AI-driven compliance monitoring and advanced certification processes |
| Technological Advancements | Expansion of AI-controlled and autonomous robotic cleaning systems |
| Industry Adoption | Widespread integration in ambulatory care, dental, and home healthcare |
| Supply Chain and Sourcing | Diversification with sustainable, locally sourced disinfection solutions |
| Market Competition | Rise of AI-powered sterilization startups and eco-friendly disinfectant brands |
| Market Growth Drivers | Demand for smart monitoring, predictive analytics, and green sterilization |
| Sustainability and Energy Efficiency | Adoption of biodegradable cleaning agents and energy-efficient sterilization |
| Integration of Smart Monitoring | Full-scale IoT-enabled sterilization with real-time compliance tracking |
| Advancements in Product Innovation | AI-assisted automated disinfection, smart sensors, and eco-conscious technologies |
Surge in awareness, government initiatives, and strong healthcare infrastructure drive the monkeypox treatment market in the United States Growing cases and the immediate response from health organizations such as the CDC and FDA are the main growth engines.
Demand is rising, and pharmaceutical companies are investing in antiviral drugs, vaccines and monoclonal antibody treatments. Major biotech firms and malicious research facilities also drive innovation. Moreover, partnerships between public institutions and private entities are making safe treatments and preventative measures more accessible. Increasing healthcare spending and insurance coverage for the treatment of infectious diseases are other factors that promote the growth of the market.
| Country | CAGR (2025 to 2035) |
|---|---|
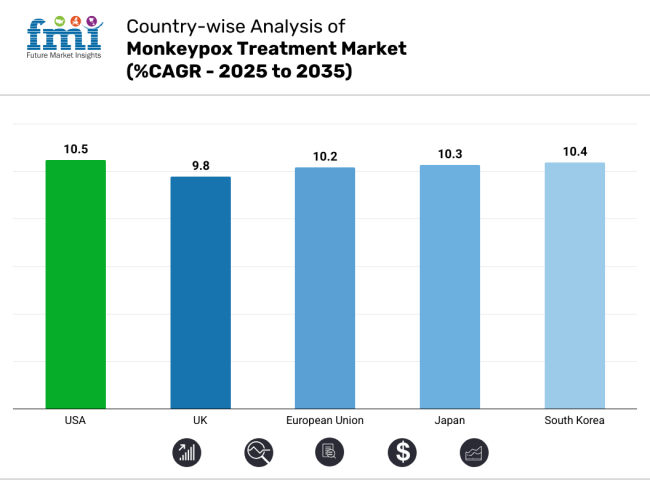
| USA | 10.5% |
The United Kingdom is the leading country in monkeypox treatment market based on vibrant government policies, advanced medical research and efficient health care system. The NHS has developed plans for selective vaccination and post-exposure treatments to control the spread of infection.
Market growth is expected to be driven by ongoing research and development in antiviral drugs, and regulatory approvals for new medications. Uplift into the market is also contributed through augmented funding for infectious diseases management, together with strong association among pharmaceutical companies and public health organization. Moreover, increasing awareness campaigns and public health initiatives are making treatments more accessible.
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 9.8% |
The European Union market for monkeypox treatment is growing, and this is in large part due to the collaborative efforts of both the European Medicines Agency (EMA) and European health bodies. Such measures are by now part of the new normal, the response of countries including Germany, France and Italy to greater uptake of vaccines and antivirals to mitigate outbreaks.
Biotech companies are establishing strong research collaborative partnerships with academic institutions to bring drugs to market faster. Moreover, the mass procurement of vaccines by government bodies guarantees extensive immunization, further affecting the market growth. Public-private partnerships are bolstering treatment accessibility, and developments in molecular diagnostics facilitate early detection and intervention strategies.
| Region | CAGR (2025 to 2035) |
|---|---|
| European Union (EU) | 10.2% |
The Japanese monkeypox treatment market is poised to grow through robust government initiatives, advancements in technology, and rising investments for the management of infectious diseases. These include rapid response strategies such as mass immunization campaigns and novel therapeutic drug development projects.
Japanese pharmaceutical companies are teaming with foreign biotech firms to discover effective antiviral medicines. As preventive healthcare and advanced molecular diagnostic tools are becoming widely available, the focus on early detection and timely therapeutic approaches is translating to improved disease-free survival. Moreover, in Japan, regulatory authorities are fast-tracking approvals for novel vaccines and therapeutic strategies, thereby driving market growth.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 10.3% |
The monkeypox treatment market in South Korea is developing owing to the proactive policies of the government, high healthcare expenditure, and rapid technological integration in the healthcare sector. The nation has also poured resources into the research and development of antiviral treatments, vaccines and rapid diagnostic tools.
The presence of leading biotech companies and research institutions are fueling innovation regarding monkeypox therapeutics. Public awareness campaigns are effective and vaccination. Moreover, the availability of free telemedicine services and digital healthcare platforms is enabling patients to receive prompt diagnosis and treatment, thus promoting market expansion.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 10.4% |
Growing occurrences of monkeypox outbreaks, boosters in awareness, and vaccination campaigns led by the governments are driving the market for monkeypox treatment. Based on type the market is segmented into prophylactic vaccines and therapeutic treatments. The vaccines most in use to help curb transmissions, including Jynneos and ACAM2000, are prophylactics utilized in high-risk populations to prevent the scourge of the virus.
Therapeutic agents such as Tecovirimat, Brincidofovir, and Cidofovir are critical in severe or immunocompromised cases. The increase in approvals of new antiviral treatments from regulatory agencies is likely to lead to innovations in new drugs in the market along with their availability in developed and underdeveloped countries.
This disease can include a sub-classification of monkeypox route of administration treatment market, which shows the popularity of oral and injectable route of administration in Monkeypox medication. The oral option (e.g. Tecovirimat capsule) allows for ease of use and improved patient compliance and universal use in outpatient care.
On the other hand, injectable antivirals such as Brincidofovir and Cidofovir are reserved for hospital use only, as patients with severe infections will need immediate treatment. The growing emphasis on the development of long-acting injectables and combination therapies is projected to enhance effectiveness of treatment and patient outcomes. Moreover, continuous clinical trials, as well as government funding for next-generation antivirals, are also accelerating treatment administration technology development.
The market for monkeypox treatment is segmented by distribution channel into retail pharmacies, hospital pharmacies, and online pharmacies, ensuring availability of vaccines and therapeutics to the general population. Hospital pharmacies are the principal distribution channel, serving critical and inpatients needing urgent medical care.
Gradually, retail pharmacies are complementing vaccination and oral antivirals services and enhancing the availability in urban and semi-urban spaces. On the other hand, online pharmacies are growing significantly, providing delivery of medicines to the doorstep, online consultations, and more convenience to patients.
Government-led vaccine distribution programs and partnerships between pharmaceutical companies and healthcare organizations are also driving the expansion of monkeypox treatment availability across multiple distribution pathways.
The market for monkeypox treatment is experiencing substantial growth as a result of the growing number of reported cases and the rising emphasis on antiviral medications, vaccines, and supportive care. Pharmaceutical firms are heavily investing in research and development to launch effective treatments, advance diagnostic methods, and increase patient outcomes.
Governments and healthcare institutions are playing a proactive role in expanding vaccination campaigns and stockpiling critical drugs to counteract potential outbreaks. Also, progress in biotechnology and immunology is enabling the creation of targeted therapies that enhance the rate of recovery as well as minimize infections. The need for efficient antiviral drugs, coupled with approvals of new vaccines, is driving the competition of the market.
Market Share Analysis by Company
| Company Name | Key Offerings/Activities |
|---|---|
| SIGA Technologies | Leading provider of antiviral medications, including TPOXX (tecovirimat), a primary treatment option for Monkeypox. |
| Bavarian Nordic | Manufacturer of the Jynneos vaccine, a key preventive solution against Monkeypox and other orthopoxvirus infections. |
| Chimerix | Develops oral antiviral treatments, focusing on next-generation therapies for smallpox-related illnesses, including Monkeypox. |
| Emergent BioSolutions | Specializes in medical countermeasures, including vaccines and immune therapeutics for Monkeypox prevention and treatment. |
| Tonix Pharmaceuticals | Focuses on developing live virus vaccines aimed at strengthening immunity against emerging infectious diseases like Monkeypox. |
Key Company Insights
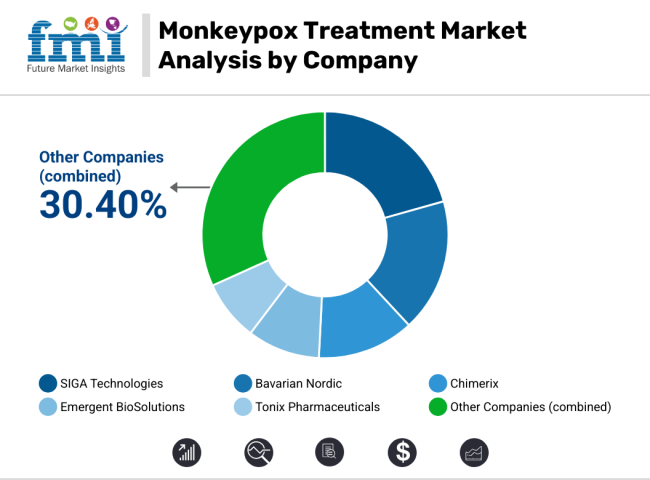
SIGA Technologies (22-26%)
SIGA Technologies dominates the monkeypox treatment industry through its antiviral drug TPOXX (tecovirimat), an approved drug against smallpox that is progressively being used in the case of Monkeypox. SIGA invests heavily in clinical research, widening the scope of using it to handle serious cases.
Government contracts, stockpiles, and world distribution deals cement its market stronghold. Ongoing studies into combinations of treatments as well as prolonging the scope of treatment widen SIGA's market base further. The firm is also collaborating with health authorities to increase accessibility and affordability in outbreak-vulnerable areas.
Bavarian Nordic (18-22%)
Bavarian Nordic is another important player in the market for monkeypox treatments, with its Jynneos vaccine being used for immunization against Monkeypox. They are involved in supply contracts with governments and international health organizations.
Research is being conducted to improve the effectiveness of vaccines, as well as their stability for storage and scalability of production. Where It Stands Bavarian Nordic is laying the groundwork by cultivating alliances with healthcare providers and public health organizations worldwide. Investments in next-generation vaccine technologies are also boosting the company’s competitive edge.
Chimerix (12-16%)
Chimerix is an antiviral pharmaceutical company focused on creating new solutions for orthopoxvirus infections such as monkeypox. Its pipeline also has some promising antiviral candidates that can diminish disease severity and transmission. The company works with government agencies and research institutions to speed up drug approval processes and broaden distribution networks.
Using advanced biotechnology, Chimerix is designing better oral drugs, which makes it easy for the patients. Its focus around broad-spectrum antivirals also makes it one of the leading contenders in the market.
Emergent BioSolutions (8-12%)
Emergent BioSolutions is a market leader in medical countermeasures, particularly monkeypox vaccines and immune therapeutics. It collaborates with key global health organizations and develops strong relationships with regulatory authorities to fast track approvals of their offerings.
Its manufacturing and large-scale distribution know-how enables quick outbreak response. With an eye on growing demand, Emergent BioSolutions is investing in sophisticated vaccine development and broadening its production capabilities. The company also collaborates with governments to improve preparedness plans and advance the overall Monkeypox treatment ecosystem.
Tonix Pharmaceuticals (6-10%)
Tonix Pharmaceuticals is another type of player on the monkeypox therapeutic landscape, building a live virus vaccines that targets enhanced immune responses. We are conducting extensive research on the development of next-generation vaccines that have long-term immunity and cross-protection against related viruses.
This innovative approach employs modified live virus platforms to augment vaccine effectiveness. Tonix works with biotech companies and research organizations to increase efficiency in clinical trials and regulatory processes. It would be a great contender for new wave of monkeypox treatments with its commitment to new immunization methods.
Other Key Players (30-40% Combined)
Several other pharmaceutical and biotech firms are contributing to the growth of the Monkeypox treatment market. These companies focus on antiviral research, vaccine production, and immune system modulation. Key players include:
The competitive landscape of the monkeypox treatment market is dynamic, with ongoing research, innovation, and strategic partnerships driving advancements in treatment options. Companies are increasingly focusing on expanding their portfolios, enhancing global accessibility, and leveraging cutting-edge technologies to address the growing demand for effective Monkeypox treatment and prevention solutions.
The overall market size for monkeypox treatment market was USD 114.06 million in 2025.
The monkeypox treatment market expected to reach USD 295.85 million in 2035.
Rising monkeypox cases, increasing government initiatives, growing antiviral drug development, expanding vaccine distribution, and advancements in diagnostic technologies will drive market demand.
The top 5 countries which drives the development of monkeypox treatment market are USA, UK, Europe Union, Japan and South Korea.
Expanding distribution channels driving market growth to command significant share over the assessment period.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 5: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 9: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 10: Latin America Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 11: Latin America Market Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 12: Latin America Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 13: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Europe Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 15: Europe Market Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 16: Europe Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 17: Asia Pacific Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 18: Asia Pacific Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 19: Asia Pacific Market Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 20: Asia Pacific Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 21: MIDDLE EAST AND AFRICA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: MIDDLE EAST AND AFRICA Market Value (US$ Million) Forecast by Treatment Type, 2018 to 2033
Table 23: MIDDLE EAST AND AFRICA Market Value (US$ Million) Forecast by Route of Administration, 2018 to 2033
Table 24: MIDDLE EAST AND AFRICA Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 4: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 5: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 9: Global Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 10: Global Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 11: Global Market Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 12: Global Market Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 13: Global Market Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 14: Global Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 15: Global Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 16: Global Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 17: Global Market Attractiveness by Treatment Type, 2023 to 2033
Figure 18: Global Market Attractiveness by Route of Administration, 2023 to 2033
Figure 19: Global Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 20: Global Market Attractiveness by Region, 2023 to 2033
Figure 21: North America Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 22: North America Market Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 23: North America Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 24: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 25: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 26: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 27: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 28: North America Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 29: North America Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 30: North America Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 31: North America Market Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 34: North America Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 35: North America Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 36: North America Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 37: North America Market Attractiveness by Treatment Type, 2023 to 2033
Figure 38: North America Market Attractiveness by Route of Administration, 2023 to 2033
Figure 39: North America Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 40: North America Market Attractiveness by Country, 2023 to 2033
Figure 41: Latin America Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 42: Latin America Market Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 43: Latin America Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 44: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 45: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 48: Latin America Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 49: Latin America Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 50: Latin America Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 51: Latin America Market Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 52: Latin America Market Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 53: Latin America Market Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 54: Latin America Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 55: Latin America Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 56: Latin America Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 57: Latin America Market Attractiveness by Treatment Type, 2023 to 2033
Figure 58: Latin America Market Attractiveness by Route of Administration, 2023 to 2033
Figure 59: Latin America Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 60: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 61: Europe Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 62: Europe Market Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 63: Europe Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 64: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 65: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 66: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 67: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 68: Europe Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 69: Europe Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 70: Europe Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 71: Europe Market Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 72: Europe Market Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 73: Europe Market Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 74: Europe Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 75: Europe Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 76: Europe Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 77: Europe Market Attractiveness by Treatment Type, 2023 to 2033
Figure 78: Europe Market Attractiveness by Route of Administration, 2023 to 2033
Figure 79: Europe Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 80: Europe Market Attractiveness by Country, 2023 to 2033
Figure 81: Asia Pacific Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 82: Asia Pacific Market Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 83: Asia Pacific Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 84: Asia Pacific Market Value (US$ Million) by Country, 2023 to 2033
Figure 85: Asia Pacific Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 86: Asia Pacific Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 87: Asia Pacific Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 88: Asia Pacific Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 89: Asia Pacific Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 90: Asia Pacific Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 91: Asia Pacific Market Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 92: Asia Pacific Market Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 93: Asia Pacific Market Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 94: Asia Pacific Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 95: Asia Pacific Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 96: Asia Pacific Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 97: Asia Pacific Market Attractiveness by Treatment Type, 2023 to 2033
Figure 98: Asia Pacific Market Attractiveness by Route of Administration, 2023 to 2033
Figure 99: Asia Pacific Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 100: Asia Pacific Market Attractiveness by Country, 2023 to 2033
Figure 101: MIDDLE EAST AND AFRICA Market Value (US$ Million) by Treatment Type, 2023 to 2033
Figure 102: MIDDLE EAST AND AFRICA Market Value (US$ Million) by Route of Administration, 2023 to 2033
Figure 103: MIDDLE EAST AND AFRICA Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 104: MIDDLE EAST AND AFRICA Market Value (US$ Million) by Country, 2023 to 2033
Figure 105: MIDDLE EAST AND AFRICA Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 106: MIDDLE EAST AND AFRICA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 107: MIDDLE EAST AND AFRICA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 108: MIDDLE EAST AND AFRICA Market Value (US$ Million) Analysis by Treatment Type, 2018 to 2033
Figure 109: MIDDLE EAST AND AFRICA Market Value Share (%) and BPS Analysis by Treatment Type, 2023 to 2033
Figure 110: MIDDLE EAST AND AFRICA Market Y-o-Y Growth (%) Projections by Treatment Type, 2023 to 2033
Figure 111: MIDDLE EAST AND AFRICA Market Value (US$ Million) Analysis by Route of Administration, 2018 to 2033
Figure 112: MIDDLE EAST AND AFRICA Market Value Share (%) and BPS Analysis by Route of Administration, 2023 to 2033
Figure 113: MIDDLE EAST AND AFRICA Market Y-o-Y Growth (%) Projections by Route of Administration, 2023 to 2033
Figure 114: MIDDLE EAST AND AFRICA Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 115: MIDDLE EAST AND AFRICA Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 116: MIDDLE EAST AND AFRICA Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 117: MIDDLE EAST AND AFRICA Market Attractiveness by Treatment Type, 2023 to 2033
Figure 118: MIDDLE EAST AND AFRICA Market Attractiveness by Route of Administration, 2023 to 2033
Figure 119: MIDDLE EAST AND AFRICA Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 120: MIDDLE EAST AND AFRICA Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market
Water Treatment Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Equipment Market Size and Share Forecast Outlook 2025 to 2035
Burns Treatment Market Overview – Growth, Demand & Forecast 2025 to 2035
CRBSI Treatment Market Insights - Growth, Trends & Forecast 2025 to 2035
Water Treatment Polymers Market Growth & Demand 2025 to 2035
Water Treatment System Market Growth - Trends & Forecast 2025 to 2035
Algae Treatment Chemical Market Growth – Trends & Forecast 2024-2034
Water Treatment Chemical Market Growth – Trends & Forecast 2024-2034
Anemia Treatment Market Analysis - Growth & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA