The Mono Rapid Testing Market is estimated to be valued at USD 1.4 billion in 2025 and is projected to reach USD 3.1 billion by 2035, registering a compound annual growth rate (CAGR) of 8.2% over the forecast period.
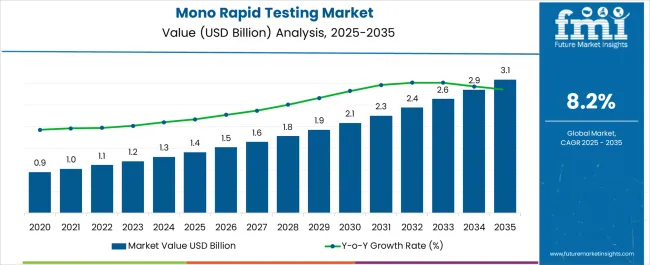
| Metric | Value |
|---|---|
| Mono Rapid Testing Market Estimated Value in (2025 E) | USD 1.4 billion |
| Mono Rapid Testing Market Forecast Value in (2035 F) | USD 3.1 billion |
| Forecast CAGR (2025 to 2035) | 8.2% |
The Mono Rapid Testing market is experiencing strong growth, driven by the increasing prevalence of infectious diseases and the need for rapid, accurate diagnostic solutions. Adoption is being supported by the growing focus on point-of-care testing and the demand for timely detection in hospitals, clinics, and diagnostic laboratories. Lateral flow assay kits have emerged as a preferred testing method due to their simplicity, portability, and ability to deliver quick results without extensive laboratory infrastructure.
Advances in immunoassay technology and bio-sensing capabilities are enhancing sensitivity and specificity, improving diagnostic confidence. Increasing awareness among healthcare providers and patients regarding early detection and disease management is further driving market expansion. Government initiatives, funding for public health programs, and rising healthcare expenditures in emerging economies are supporting widespread adoption.
The growing trend of integrating rapid diagnostic testing into routine healthcare workflows, coupled with the emphasis on reducing turnaround times for infectious disease detection, positions the market for sustained growth Continuous innovation in assay design, sample processing, and accuracy improvements is expected to create further opportunities in the coming decade.
The mono rapid testing market is segmented by product type, application type, end user, and geographic regions. By product type, mono rapid testing market is divided into Lateral Flow Assay Kit and Agglutination Assay Kits. In terms of application type, mono rapid testing market is classified into Infectious Diseases Testing, Substance Abuse Testing, and Others.
Based on end user, mono rapid testing market is segmented into Hospitals, Clinics, Homecare Setting, Research Centers, and Diagnostics Centers. Regionally, the mono rapid testing industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
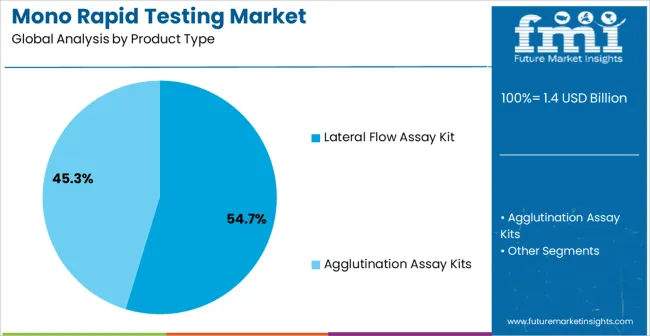
The lateral flow assay kit segment is projected to hold 54.7% of the market revenue in 2025, establishing it as the leading product type. Growth is being driven by the convenience and rapid turnaround time offered by these kits, which allow for near-immediate detection of infectious agents without the need for specialized equipment. Their portability and ease of use make them suitable for hospital wards, clinics, and remote testing locations.
Improvements in assay sensitivity and specificity, along with the development of multiplex testing capabilities, have enhanced clinical reliability and adoption. Regulatory approvals and standardization of testing protocols have further reinforced confidence among healthcare providers.
Cost-effectiveness, minimal training requirements, and ability to integrate into routine testing workflows are additional factors supporting their market leadership As demand for fast, accurate, and scalable testing solutions continues to rise, lateral flow assay kits are expected to maintain their dominance, underpinned by continuous technological advancements and increased public health awareness.
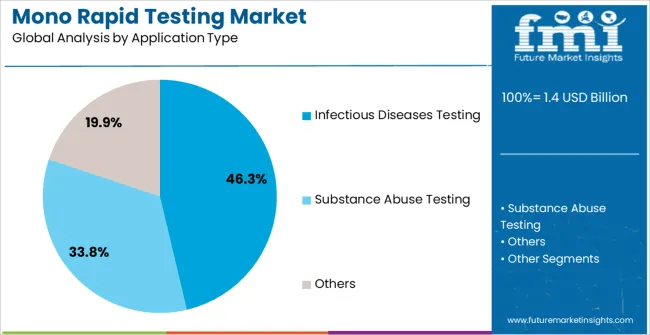
The infectious diseases testing application segment is expected to account for 46.3% of the market revenue in 2025, making it the leading application. Growth in this segment is being driven by the high prevalence of diseases such as mononucleosis, influenza, and other viral and bacterial infections, which require rapid and reliable diagnosis.
The use of lateral flow assays enables timely detection, improving patient outcomes and reducing the risk of disease transmission. Increasing public health initiatives, funding for diagnostic programs, and awareness campaigns have supported adoption in both developed and emerging markets.
Integration with hospital workflows and point-of-care testing protocols allows for immediate clinical decision-making and streamlined patient management As the demand for rapid diagnostics continues to rise, especially in the context of infectious disease outbreaks and routine screenings, the infectious diseases testing segment is expected to remain a primary driver of market growth, supported by technological advancements and increased healthcare investments.
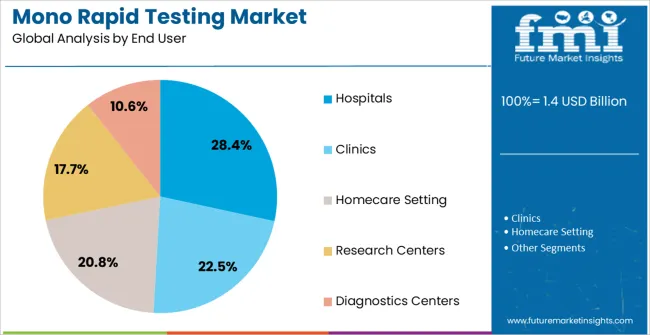
The hospitals end-user segment is projected to hold 28.4% of the market revenue in 2025, establishing it as the leading end-use category. Hospitals are increasingly adopting mono rapid testing solutions to ensure fast, accurate diagnosis and efficient patient management. The demand is driven by the need to reduce patient waiting times, improve workflow efficiency, and minimize the spread of infectious diseases within clinical settings.
Integration with hospital laboratories and point-of-care testing protocols allows rapid deployment of test results for immediate clinical decision-making. Hospitals benefit from the scalability, reliability, and user-friendly nature of lateral flow assay kits, which can be easily implemented in various departments, including emergency care, outpatient services, and intensive care units.
Growing emphasis on public health compliance, disease surveillance, and operational efficiency is reinforcing adoption As hospitals continue to prioritize timely diagnosis, infection control, and workflow optimization, this segment is expected to maintain its leadership position, supported by increasing investments in rapid testing infrastructure and healthcare technology innovations.
Mono rapid testing is a type of point of care testing for the qualitative detection of mononucleosis heterophile antibodies in the whole blood. This test is only done for the diagnosis of infectious mononucleosis in the blood sample. Infectious mononucleosis is an acute lymphoproliferative disease. This disease is caused by Epstein-Barr virus (EBV) and it is non-recognizable disease during its early stages.
The diagnosis of infectious mononucleosis is done by mono rapid test kits. This diagnosis is based on the on the evaluation of characteristic serological, clinical, and hematological changes in the blood sample. Physicians suggest to perform the mono rapid test for the detection of infectious mononucleosis if the patient is suffering from fever, pharyngitis, and cervical lymphadenopathy for more than 4 weeks.
The result of mono rapid testing can be analyzed in few minute. There is no age restriction to perform the mono rapid testing. The mono rapid test kit includes a cassette, dropper, and reagents such as mono developer solution.
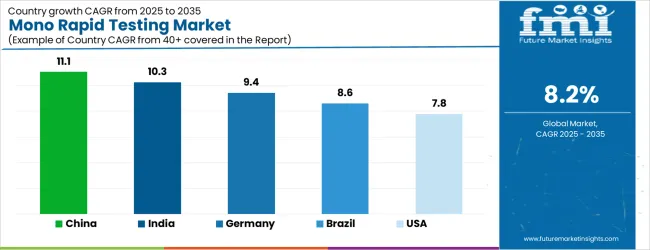
| Country | CAGR |
|---|---|
| China | 11.1% |
| India | 10.3% |
| Germany | 9.4% |
| Brazil | 8.6% |
| USA | 7.8% |
| UK | 7.0% |
| Japan | 6.2% |
The Mono Rapid Testing Market is expected to register a CAGR of 8.2% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 11.1%, followed by India at 10.3%. Developed markets such as Germany, France, and the U.K. continue to expand steadily, while the U.S. is likely to grow at consistent rates. Japan posts the lowest CAGR at 6.2%, yet still underscores a broadly positive trajectory for the global Mono Rapid Testing Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 9.4%. The U.S. Mono Rapid Testing Market is estimated to be valued at USD 524.2 million in 2025 and is anticipated to reach a valuation of USD 524.2 million by 2035. Sales are projected to rise at a CAGR of 0.0% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 76.6 million and USD 43.0 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 1.4 Billion |
| Product Type | Lateral Flow Assay Kit and Agglutination Assay Kits |
| Application Type | Infectious Diseases Testing, Substance Abuse Testing, and Others |
| End User | Hospitals, Clinics, Homecare Setting, Research Centers, and Diagnostics Centers |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
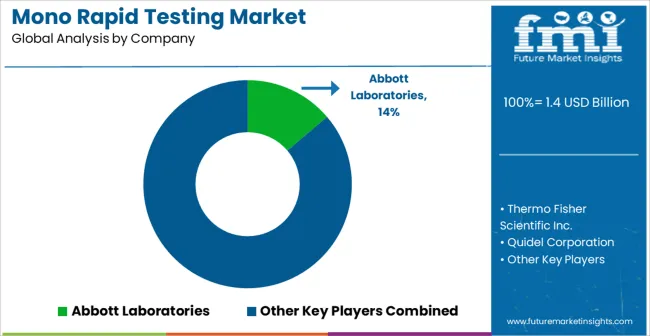
| Key Companies Profiled | Abbott Laboratories, Thermo Fisher Scientific Inc., Quidel Corporation, Becton, Dickinson and Company, F. Hoffmann-La Roche Ltd, Bio-Rad Laboratories, Inc., Siemens Healthineers AG, Danaher Corporation, PerkinElmer, Inc., Hologic, Inc., OraSure Technologies, Inc., Meridian Bioscience, Inc., and Chembio Diagnostics, Inc. |
The global mono rapid testing market is estimated to be valued at USD 1.4 billion in 2025.
The market size for the mono rapid testing market is projected to reach USD 3.1 billion by 2035.
The mono rapid testing market is expected to grow at a 8.2% CAGR between 2025 and 2035.
The key product types in mono rapid testing market are lateral flow assay kit and agglutination assay kits.
In terms of application type, infectious diseases testing segment to command 46.3% share in the mono rapid testing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Rapid RNA Testing Kits Market Trends- Growth & Forecast 2025 to 2035
Rapid Antigen Testing Market - Demand, Growth & Forecast 2025 to 2035
Rapid Hepatitis Testing Market – Demand & Forecast 2025 to 2035
Rapid Coagulation Testing Market
Rapid Microbiology Testing Market Forecast Outlook 2025 to 2035
Malaria Ag Rapid Testing Market - Growth & Forecast 2025 to 2035
Candida Vaginitis Rapid Testing Market
Rapid U-drills Market Size and Share Forecast Outlook 2025 to 2035
Rapid Test Cards Market Size and Share Forecast Outlook 2025 to 2035
Monochrome Outdoor LED Display Market Size and Share Forecast Outlook 2025 to 2035
Monoaluminum Phosphate Market Size and Share Forecast Outlook 2025 to 2035
Monofilament Mesh Filter Bags Market Size and Share Forecast Outlook 2025 to 2035
Rapid Prototyping Materials Market Size and Share Forecast Outlook 2025 to 2035
Monolithic UPS Market Size and Share Forecast Outlook 2025 to 2035
Rapid Test Readers Market Size and Share Forecast Outlook 2025 to 2035
Monocrystalline Solar Cell Market Size and Share Forecast Outlook 2025 to 2035
Monoclonal Gammopathy of Undetermined Significance (MGUS) Management Market Size and Share Forecast Outlook 2025 to 2035
Monostarch Phosphate Market Size and Share Forecast Outlook 2025 to 2035
Monoethylene Glycol MEG Market Size and Share Forecast Outlook 2025 to 2035
Rapid Strength Concrete Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA