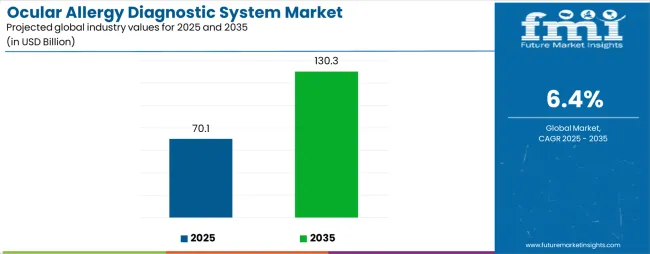
The Ocular Allergy Diagnostic System Market is estimated to be valued at USD 70.1 billion in 2025 and is projected to reach USD 130.3 billion by 2035, registering a compound annual growth rate (CAGR) of 6.4% over the forecast period.
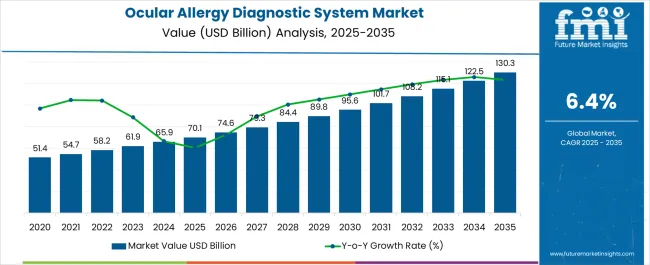
| Metric | Value |
|---|---|
| Ocular Allergy Diagnostic System Market Estimated Value in (2025 E) | USD 70.1 billion |
| Ocular Allergy Diagnostic System Market Forecast Value in (2035 F) | USD 130.3 billion |
| Forecast CAGR (2025 to 2035) | 6.4% |
The Ocular Allergy Diagnostic System market is experiencing steady growth, driven by the increasing prevalence of allergic eye diseases such as conjunctivitis and keratoconjunctivitis across diverse age groups. Rising exposure to allergens including pollen, dust, and pollution is creating higher demand for timely and accurate diagnostic solutions. Advancements in medical technology and the growing emphasis on early disease detection are contributing significantly to the adoption of these systems.
Non-invasive methods that provide quick, accurate, and patient-friendly diagnostics are emerging as a key trend, lowering discomfort while enhancing compliance. Healthcare providers are also prioritizing cost-effective diagnostic platforms that integrate seamlessly into clinical workflows and support data-driven treatment planning. In addition, rising awareness about ocular health and the availability of specialized diagnostic devices in both developed and emerging markets are strengthening market dynamics.
With governments and private organizations increasing investments in healthcare infrastructure, the availability and adoption of advanced diagnostic tools are expanding As ocular allergies become more prevalent and patient expectations evolve, the market is positioned for sustained growth with innovation and accessibility acting as major driving forces.
The ocular allergy diagnostic system market is segmented by type, end user, and geographic regions. By type, ocular allergy diagnostic system market is divided into Non-Invasive Diagnostics Methods and Invasive Diagnostics Methods. In terms of end user, ocular allergy diagnostic system market is classified into Hospitals, Eye Clinics, and Diagnostic Centers. Regionally, the ocular allergy diagnostic system industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
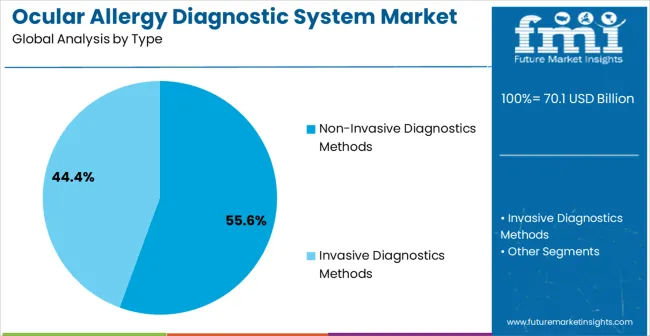
The non-invasive diagnostics methods segment is projected to account for 55.6% of the market revenue in 2025, making it the leading type segment. Growth in this segment is being driven by the preference for techniques that minimize patient discomfort while providing reliable and rapid results. Non-invasive approaches eliminate the need for invasive sampling procedures, thereby improving patient safety and acceptance.
These methods also enable repeated testing, which is critical for monitoring chronic allergic conditions and evaluating treatment effectiveness over time. The integration of advanced imaging technologies and digital platforms further enhances diagnostic accuracy and facilitates real-time assessment in clinical settings. Healthcare providers are increasingly adopting these solutions due to their efficiency, reduced risk of complications, and compatibility with modern electronic medical record systems.
The scalability of non-invasive diagnostics also allows widespread adoption across various healthcare environments, from specialized clinics to primary care facilities As technological innovations continue to refine precision and accessibility, non-invasive diagnostics methods are expected to remain the dominant type, reinforcing their role as the backbone of ocular allergy diagnostics.
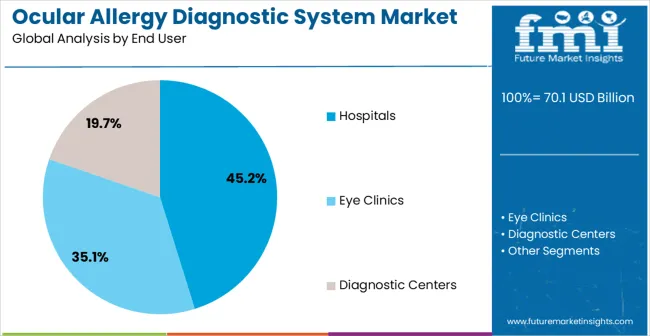
The hospitals segment is expected to hold 45.2% of the market revenue in 2025, establishing itself as the leading end user. Growth in this segment is being supported by the rising number of patients seeking diagnosis and treatment for ocular allergies in hospital settings, where access to advanced diagnostic equipment and specialized care is readily available. Hospitals provide comprehensive diagnostic services that ensure timely identification of allergic conditions, which is critical for preventing complications and improving patient outcomes.
The presence of multidisciplinary teams, including ophthalmologists and allergists, enhances the effectiveness of hospital-based diagnostics. Furthermore, hospitals are increasingly investing in advanced diagnostic platforms that integrate seamlessly with electronic health records and treatment planning systems, enabling more coordinated care.
Rising patient volumes, government funding, and insurance coverage for diagnostic services further strengthen the role of hospitals in this market As demand for high-quality and reliable allergy diagnostics continues to rise, hospitals are expected to retain their leadership position, driven by infrastructure advantages, skilled professionals, and the ability to deliver comprehensive care.
An ocular allergy is a group of hypersensitivity reactions that affect the conjunctiva and eyelids. Diagnosis of ocular allergy depends on symptoms and clinical history, with the support of in-vitro and in-vivo tests for identification of specific allergen. During the diagnosis process of ocular allergy, the allergen is considered as primary cause for the symptoms.
Allergan investigations are not required in cases where symptom are resolved with symptomatic treatment. But, in many cases specific tests (like conjunctival allergen provocation test) are used for identifying IgE mediated sensitization. Others techniques include ocular surface evaluation, ocular sampling and tear biomarkers and imaging techniques.
In the ocular surface evaluation, tear film is measured because the chronic ocular allergy is causative of dry eyes. The tear film is measured by invasive breakup time, Schirmer test and tear osmolarity. Imaging technologies have been widely used in the diagnosis of ocular allergy severity and follow-up of ocular surface disorders.
The application imaging ocular allergy diagnostic system technologies help to quantify the extent of inflammation and efficacy of anti-allergy drugs. In-vivo confocal microscopy is a noninvasive ocular allergy diagnostic technology used for microstructural analysis of cornea at a cellular level. Other non-invasive techniques include in-vivo laser scanning CM and meibography for evaluation of microscopic anatomy of eyes.
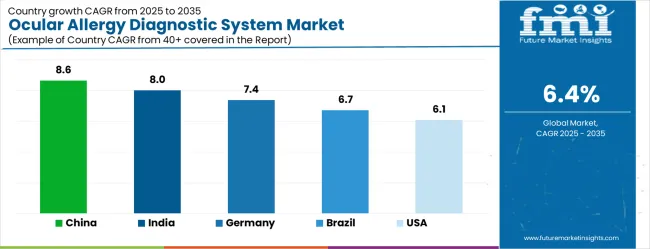
| Country | CAGR |
|---|---|
| China | 8.6% |
| India | 8.0% |
| Germany | 7.4% |
| Brazil | 6.7% |
| USA | 6.1% |
| UK | 5.4% |
| Japan | 4.8% |
The Ocular Allergy Diagnostic System Market is expected to register a CAGR of 6.4% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 8.6%, followed by India at 8.0%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Japan posts the lowest CAGR at 4.8%, yet still underscores a broadly positive trajectory for the global Ocular Allergy Diagnostic System Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 7.4%.
The USA Ocular Allergy Diagnostic System Market is estimated to be valued at USD 24.6 billion in 2025 and is anticipated to reach a valuation of USD 24.6 billion by 2035. Sales are projected to rise at a CAGR of 0.0% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 3.2 billion and USD 2.3 billion respectively in 2025.
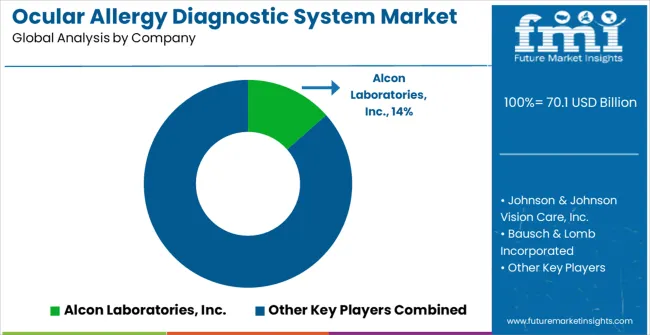
| Item | Value |
|---|---|
| Quantitative Units | USD 70.1 Billion |
| Type | Non-Invasive Diagnostics Methods and Invasive Diagnostics Methods |
| End User | Hospitals, Eye Clinics, and Diagnostic Centers |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
| Key Companies Profiled | Alcon Laboratories, Inc., Johnson & Johnson Vision Care, Inc., Bausch & Lomb Incorporated, Allergan, Inc., Novartis AG, Pfizer Inc., Sanofi S.A., Roche Holding AG, Abbott Medical Optics Inc., Merck & Co., Inc., Regeneron Pharmaceuticals, Inc., Santen Pharmaceutical Co., Ltd., and Abbott |
The global ocular allergy diagnostic system market is estimated to be valued at USD 70.1 billion in 2025.
The market size for the ocular allergy diagnostic system market is projected to reach USD 130.3 billion by 2035.
The ocular allergy diagnostic system market is expected to grow at a 6.4% CAGR between 2025 and 2035.
The key product types in ocular allergy diagnostic system market are non-invasive diagnostics methods and invasive diagnostics methods.
In terms of end user, hospitals segment to command 45.2% share in the ocular allergy diagnostic system market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Allergy Diagnostic Market Forecast and Outlook 2025 to 2035
Diagnostic X-Ray System Market – Trends & Forecast 2025 to 2035
Ocular Drug Delivery System Market – Trends & Forecast 2025 to 2035
Veterinary Allergy Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Sleep Apnea Diagnostic Systems Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
China Sleep Apnea Diagnostic Systems Market Outlook – Size, Share & Growth 2025-2035
Japan Sleep Apnea Diagnostic Systems Market Report – Size, Demand & Outlook 2025-2035
France Sleep Apnea Diagnostic Systems Market Insights – Size, Share & Demand 2025-2035
Germany Sleep Apnea Diagnostic Systems Market Trends – Growth, Innovations & Forecast 2025-2035
Automated Molecular Diagnostics Testing System Market Size and Share Forecast Outlook 2025 to 2035
United States Sleep Apnea Diagnostic Systems Market Analysis – Trends & Forecast 2025-2035
Allergy Tester Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Shipper Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Imaging Markers Market Size and Share Forecast Outlook 2025 to 2035
System-On-Package Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Imaging Services Market Size and Share Forecast Outlook 2025 to 2035
Allergy Immunotherapy Market Analysis - Size, Share & Forecast 2025 to 2035
Ocular Tuberculosis Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Systems Administration Management Tools Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Vials Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA