The pharmaceutical cold chain packaging market is projected to grow from USD 20.6 billion in 2025 to USD 83.2 billion by 2035, registering a robust CAGR of 15%. Estimated 2024 sales are valued at approximately USD 18.1 billion. The surge in biologics, mRNA vaccines, and cell & gene therapies is driving the need for insulated packaging that maintains strict temperature profiles.
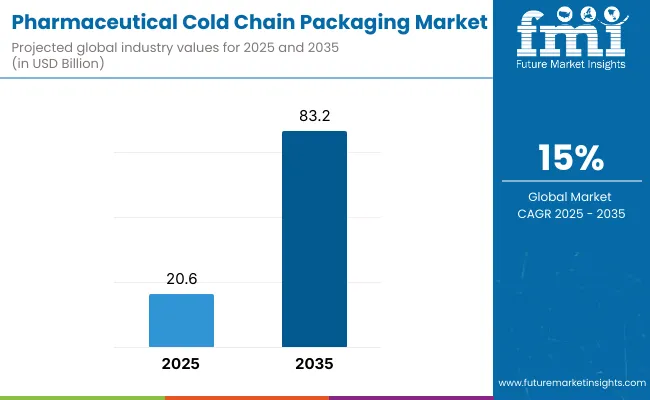
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 20.6 billion |
| Industry Value (2035F) | USD 83.2 billion |
| CAGR (2025 to 2035) | 15% |
Heightened regulatory oversight and global distribution of sensitive therapeutics are bolstering adoption. Technological advances are centering around phase-change materials (PCMs), vacuum insulation panels, and autonomous refrigeration. Integration of IoT and real-time temperature monitoring is improving traceability across multimodal routes. Modular shippers and reusable systems are also seeing greater traction for sustainability compliance. Enhanced pre-qualification protocols are enabling faster pharma-to-market timelines with reduced spoilage risks.
Future growth will stem from expanding pharmaceutical R&D pipelines, stringent FDA/EMA cold chain mandates, and rising exports from APAC and Latin America. Competition will intensify among players offering end-to-end packaging plus logistics integration. Strong focus on GDP-compliant, cost-effective packaging will shape product innovation. The market is expected to witness consolidation driven by tech-enabled differentiation and service bundling.
The below table presents the expected CAGR for the global pharmaceutical cold chain packaging market over several semi-annual periods spanning from 2025 to 2035.
| Particular | Value CAGR |
|---|---|
| H1 (2024 to 2034) | 14.4% |
| H2 (2024 to 2034) | 14.8% |
| H1 (2025 to 2035) | 14.8% |
| H2 (2025 to 2035) | 15.2% |
In the first half (H1) of the decade from 2024 to 2034, the business is predicted to surge at a CAGR of 14.4%, followed by a slightly lower growth rate of 14.8% in the second half (H2) of the same decade. Moving into the subsequent period, from H1 2025 to H2 2035, the CAGR is projected to decrease slightly to 14.8% in the first half and rise to 15.2% in the second half. In the first half (H1) the market witnessed a decrease of 100 BPS while in the second half (H2), the market witnessed an increase of 120 BPS.
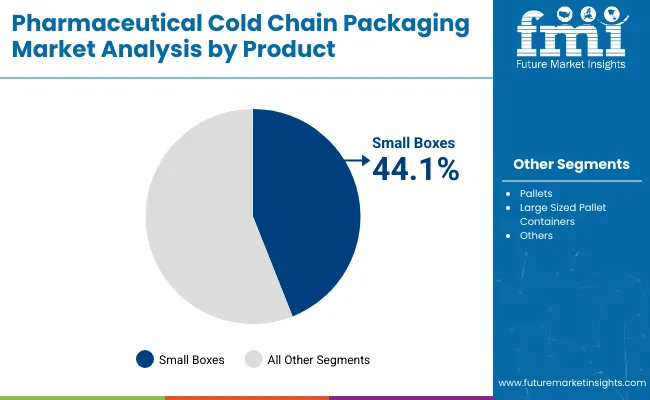
By 2025, small boxes are expected to account for 44.1% of the pharmaceutical cold chain packaging market. Their dominance can be attributed to their versatility, cost-efficiency, and suitability for small-volume, temperature-sensitive pharmaceutical products. These boxes are widely adopted for the transportation of single-dose drugs, vaccines, and clinical trial samples, especially where controlled temperature maintenance is critical.
Due to their compact dimensions, small boxes are preferred for both domestic and international distribution. Their use simplifies handling, storage, and last-mile delivery. For instance, insulated small containers are often used to ship insulin pens to pharmacies or clinics, ensuring that medications remain within the desired temperature range during transit. As a result, small boxes are positioned as the go-to format for shipments requiring temperature integrity and logistical simplicity.
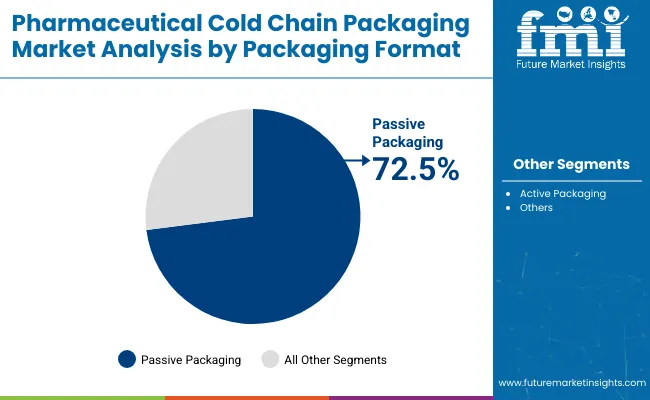
In 2025, passive packaging is projected to hold a 72.5% market share, significantly outpacing active solutions. Passive systems are being chosen due to their cost-effectiveness, ease of implementation, and ability to maintain stable temperatures without external power sources. Instead of relying on mechanical refrigeration, passive packaging employs insulating materials such as phase change materials (PCMs), gel packs, or dry ice.
Because of its reliability and simplicity, passive packaging is being favored for a wide range of cold chain applications, including the shipment of vaccines and biologics. For example, frozen gel packs or dry ice within insulated containers are commonly used to maintain strict temperature conditions during transport. Passive solutions are increasingly being adopted as they are capable of meeting ultra-cold storage requirements while offering logistical and cost advantages over active systems. As a result, the majority of cold chain shipments are being handled using passive packaging formats.
Increasing Biologics and Specialty Drugs Drive Cold Chain Packaging Requirements
Emerging markets for biologics, cell and gene treatments, and biosimilars are driving demand for pharmaceutical cold chain packaging. For these advanced medications to remain stable and effective, strict temperature control between -80°C and to 20°C must be maintained. Pharmaceutical companies rely on niche packaging solutions as their pipelines of biologics increase to prevent degradation in transit and storage.
Increased reliance on biosimilars requires more reliable cold chain logistics in turn. Beyond this, packaging manufacturers are required to innovate through high-performance insulating materials, phase change material (PCMs), and internet of things (IoT) based monitoring solutions to ensure the product integrity all the way from manufacturing to storage to delivery for cell and gene therapies, which rely on live cells.
Need for Pharmaceutical Cold Chain Packaging to Comply With Stringent Regulations
Stringent temperature monitoring and validation regulations for pharmaceutical cold chain logistics are imposed by regulatory agencies like the FDA, EMA, WHO, and IATA. To ensure medication efficacy and patient safety, these agencies mandate manufacturers, distributors, and logistics providers to maintain precise temperature conditions. Companies invest heavily in advanced cold chain packaging solutions to comply with GDP and avoid product loss due to temperature variations.
The sector is being driven towards intelligent packaging solutions by regulations that also demand data gathering, real-time monitoring, and risk reduction methods. Consistent, validated, and regulatory-approved cold chain packaging solutions are the need of the hour in the current market, and pharmaceutical firms. Different packaging providers are constantly developing to address evolving compliance requirements as enforcement becomes stricter across the world.
Infrastructure Gaps in Emerging Markets May Hinder Cold Chain Packaging Demand
It is difficult to sustain the required temperatures for pharmaceutical products in developing countries due to their sometimes poor cold storage units and unreliable electricity supplies. Spoilage is higher in situations where there are poor chilled storage units, warehouses, and power backup facilities. Poor airport infrastructure, rough roads, and inefficient last-mile distribution also lead to logistical issues that cause delays in deliveries and reduce product efficacy.
Pharmaceutical companies often use third-party logistics providers (3PLs), whose services may be inconsistent in quality, leading to compliance issues and temperature excursions. Ultimately, these infrastructure limitations reduce demand for pharmaceutical cold chain packaging within developing countries by increasing costs, disrupting supply chains, and threatening product integrity. These challenges can slow growth.
| Key Investment Area | Why It’s Critical for Future Growth |
|---|---|
| Advanced Temperature Control Technologies | To enhances product performance and reduce waste during storage and shipping by providing more precise and reliable temperature control for sensitive medications. |
| Smart Packaging & IoT Integration | Will enables real-time monitoring and tracking of temperature and humidity and increasing the efficiency of compliance and logistics processes. |
| Sustainable Cold Chain Packaging Materials | Invests in recyclable, reusable, and environmentally friendly products will address growing environmental concerns without sacrificing the cold chain's integrity. |
| Automated and AI-Driven Cold Chain Logistics | Enhances the efficiency of handling shipments, tracking, minimizing human error, and facilitating faster response to possible temperature deviations. |
| Ultra-Low Temperature Storage Solutions | Aids the increasing demand for vaccinations, gene therapies, and biologics-all of which often require extremely low temperatures for secure storage and transport. |
Tier 1 companies are market leaders that have high market share in the global market. These market leaders are known for their large production capacity and diversified product range. These leaders are identified by their broad expertise in manufacturing various packaging formats and a strong presence across geography, supported by a strong consumer base.
They provide a wide range of series including recycling and manufacturing utilizing the latest technology and meeting the regulatory standards providing the highest quality. Prominent companies within tier 1 include Sonoco ThermoSafe, Cold Chain Technologies, Sealed Air Corporation and CSafe Global LLC.
Tier 2 companies include mid-size players having presence in specific regions and highly influencing the local market. These are characterized by a strong presence overseas and strong market knowledge. These market players have good technology and ensure regulatory compliance but may not have advanced technology and wide global reach. Prominent companies in tier 2 include Envirotainer Holding AB, va-Q-tec AG, EMBALL'ISO, Sofrigam SAS, Intelsius, Peli BioThermal LLC, BIOBASE Group, Intelsius, SkyCell AG, Chill-Pak and TESSOL.
Tier 3 includes the majority of small-scale companies operating at the local presence and serving niche markets. These companies are notably oriented towards fulfilling local market demands and are consequently classified within the tier 3 share segment. They are small-scale players and have limited geographical reach. Tier-3, within this context, is recognized as an unorganized market, denoting a sector characterized by a lack of extensive structure and formalization when compared to organized competitors.
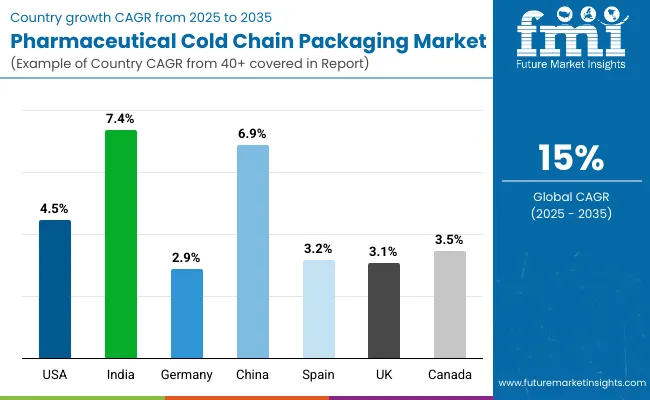
| Countries | Value CAGR (2025 to 2035) |
|---|---|
| United States | 4.5% |
| India | 7.4% |
| Germany | 2.9% |
| China | 6.9% |
| Spain | 3.2% |
| UK | 3.1% |
| Canada | 3.5% |
The section below covers the future forecast for the pharmaceutical cold chain packaging market in terms of countries. Information on key countries in several parts of the globe, including North America, Latin America, East Asia, South Asia and Pacific, Western Europe, Eastern Europe and MEA is provided. USA is anticipated to remain at the forefront in North America, with a CAGR of 4.5% through 2035. In Western Europe, Germany in anticipated to rise at a CAGR of 2.9%.
Effective packaging options are more necessary today as the number of online pharmacies and direct-to-consumer pharmaceutical providers increase in the USA Packaging must ensure the product remains safe across longer shipping timeframes because an increasing number of individuals are purchasing prescription pharmaceuticals and other products online. That means protecting the drugs from weather conditions like varying temperatures, moisture, and damage has become important.
The purity of drugs should be maintained by tamper-evident, secure, and durable packaging. The patients also require easy packing as home delivery options are gaining traction. This ensures that drugs reach in desired condition, on time, and ready for immediate use.
Specialized packaging solutions are significantly driven by the UK's focus on biopharmaceutical innovation, particularly in the areas of gene therapy and regenerative medicine. The packaging must ensure product stability, sterility, and temperature management in transit since such treatments employ complicated biologics.
Packaging capable of protecting sensitive substances while in storage and in transit is required for innovations such as gene editing and stem cell therapies. CAR-T cell treatment packaging is an example, whereby safe, cold-chain solutions must be used in order to keep these personalized medications effective until a patient receives the treatment. The packaging manufacturers are driven by this trend to provide safer, advanced systems capable of managing new-age biopharmaceuticals.
The competitive landscape of the pharmaceutical cold chain packaging market is driven by key players focused on product innovation, sustainability and strategic partnerships for strengthening their market presence. Company and consumer manufacturers are investing in environmentally friendly materials and advanced packaging technologies to meet evolving industry demands. Mergers, acquisitions and collaboration with regional manufacturers and distribution partners are becoming more common to expand market reach and optimize supply chains.
Key Developments in Pharmaceutical Cold Chain Packaging Market
The pharmaceutical cold chain packaging market is projected to witness CAGR of 15% between 2025 and 2035.
The global pharmaceutical cold chain packaging market industry stood at USD 18.1 billion in 2024.
Global pharmaceutical cold chain packaging market industry is anticipated to reach USD 83.2 billion by 2035 end.
South Asia & Pacific is set to record a CAGR of 8.1% in assessment period.
Some of the key players operating in the pharmaceutical cold chain packaging industry are Sonoco ThermoSafe, Cold Chain Technologies, Sealed Air Corporation and CSafe Global LLC.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Pharmaceutical Cold Chain Packaging Industry Analysis in United States - Size, Share, and Forecast Outlook 2025 to 2035
Pharmaceutical Zinc Powder Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Grade Magnesium Sulfate Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Manufacturing Equipment Market Forecast and Outlook 2025 to 2035
Pharmaceutical Plastic Bottle Market Forecast and Outlook 2025 to 2035
Pharmaceutical Grade Sodium Carbonate Market Forecast and Outlook 2025 to 2035
Pharmaceutical Industry Analysis in Saudi Arabia Forecast and Outlook 2025 to 2035
Pharmaceutical Grade Sodium Chloride Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Plastic Pots Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceuticals Pouch Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Mini Batch Blender Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Continuous Manufacturing Equipment Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Liquid Prefilters Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Grade P-Toluenesulfonic Acid Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Glass Container Industry Analysis in Europe Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Container Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Sterility Testing Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceuticals Preservative Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Track and Trace Systems Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Vials Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA