The Preimplantation Genetic Testing Market is estimated to be valued at USD 934.5 million in 2025 and is projected to reach USD 2294.8 million by 2035, registering a compound annual growth rate (CAGR) of 9.4% over the forecast period.
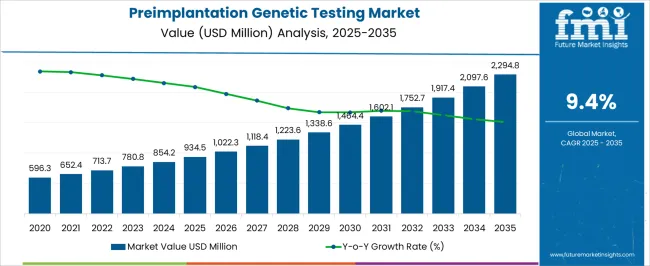
| Metric | Value |
|---|---|
| Preimplantation Genetic Testing Market Estimated Value in (2025 E) | USD 934.5 million |
| Preimplantation Genetic Testing Market Forecast Value in (2035 F) | USD 2294.8 million |
| Forecast CAGR (2025 to 2035) | 9.4% |
The Preimplantation Genetic Testing market is witnessing steady growth, driven by the increasing adoption of assisted reproductive technologies and the rising prevalence of genetic disorders among prospective parents. The demand for accurate and early detection of chromosomal abnormalities is being supported by advancements in molecular diagnostics, next-generation sequencing, and high-throughput screening techniques. Growing awareness among patients and healthcare providers about fertility options and the importance of genetic health is further accelerating market adoption.
Investments in advanced laboratory infrastructure and the integration of automated platforms for high-precision testing are improving efficiency and reliability. Regulatory support and standardized protocols for genetic testing are fostering trust and encouraging adoption in fertility clinics and maternity centers.
Additionally, the expansion of IVF services and personalized reproductive healthcare strategies is contributing to sustained market growth As technology continues to evolve and awareness increases, preimplantation genetic testing is expected to become a standard part of fertility care, providing actionable insights for clinicians and patients while reducing risks associated with genetic abnormalities.
The preimplantation genetic testing market is segmented by application type, product type, end user, and geographic regions. By application type, preimplantation genetic testing market is divided into Aneuploidy Screening, Gender Screening, Chromosomal Aberration Screening, HLA Typing, Single Gene Disorder Screening, and Others. In terms of product type, preimplantation genetic testing market is classified into Reagents, Instruments, Analyzer Software, and Accessories & Consumables. Based on end user, preimplantation genetic testing market is segmented into Fertility Clinics & Maternity Centers, Hospitals, Diagnostic Laboratories, and Academic Institutions. Regionally, the preimplantation genetic testing industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
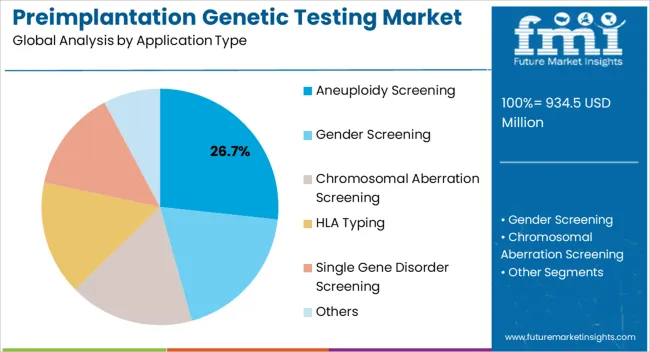
The aneuploidy screening application type is projected to hold 26.7% of the market revenue in 2025, establishing it as the leading application segment. Its growth is being driven by the critical need to identify chromosomal abnormalities in embryos prior to implantation, which improves IVF success rates and reduces the risk of miscarriage. Advanced genetic testing platforms, including high-resolution sequencing and microarray technologies, allow for rapid and accurate detection of aneuploidies.
These capabilities provide clinicians with actionable data to select viable embryos and improve patient outcomes. Integration with laboratory information management systems enhances workflow efficiency and minimizes human error.
Growing awareness among prospective parents regarding the benefits of early genetic screening has further contributed to adoption With increasing investments in fertility centers and expansion of IVF services worldwide, the aneuploidy screening segment is expected to maintain its leadership, driven by its proven clinical value and the ongoing demand for precision reproductive healthcare solutions.
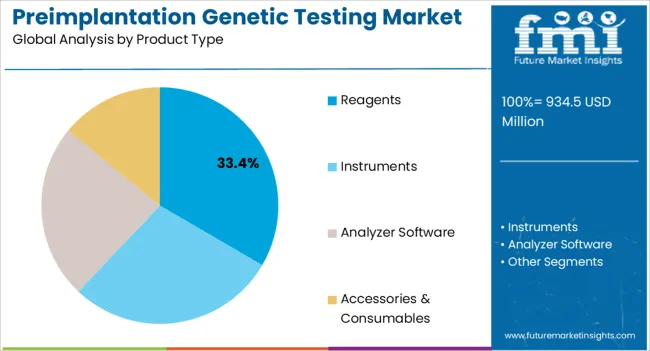
The reagents product type is anticipated to account for 33.4% of the market revenue in 2025, making it the leading product category. Growth in this segment is being driven by the need for high-quality, reliable reagents that support accurate genetic analysis in preimplantation testing. Reagents are essential for DNA amplification, sequencing, and chromosomal assessment, which ensures precise detection of genetic abnormalities.
Manufacturers are focusing on optimizing reagent formulations to improve sensitivity, reduce error rates, and enable high-throughput testing. Standardization of reagents and compatibility with automated platforms enhance laboratory efficiency and reproducibility of results.
The increasing number of IVF procedures, coupled with rising demand for personalized reproductive care, has strengthened the adoption of high-quality reagents in fertility clinics and maternity centers Continuous innovation, regulatory compliance, and focus on reproducibility are expected to support sustained growth in the reagents segment, reinforcing its position as the market leader among product types.
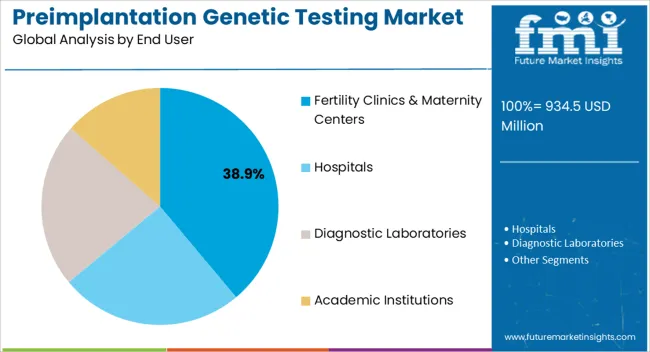
The fertility clinics and maternity centers end user segment is projected to hold 38.9% of the market revenue in 2025, making it the largest end-use category. Its growth is being driven by the increasing adoption of IVF and assisted reproductive technologies, which require comprehensive genetic testing for improved success rates. Fertility clinics and maternity centers are investing in advanced laboratory infrastructure and automated platforms to provide reliable and efficient preimplantation genetic testing services.
Clinicians are leveraging these services to enhance embryo selection, reduce miscarriage risk, and provide personalized reproductive care. Rising patient awareness about the benefits of genetic screening and early intervention is further supporting adoption.
Integration with laboratory information management systems, along with standardized testing protocols, ensures accuracy and compliance with regulatory requirements As demand for fertility services continues to rise globally, the segment is expected to maintain its leading position, driven by technological innovation, clinical effectiveness, and increasing patient preference for specialized reproductive healthcare solutions.
Preimplantation genetic testing or preimplantation genetic diagnosis is a technique in which the embryos prepared through in vitro fertilization are tested for defects before implantation. Preimplantation genetic testing enables physicians and to identify the defects present in the embryos and selectively implant healthy embryos in the uterus which increases the chances of delivering a genetically healthy baby.
Preimplantation genetic testing helps people to avoid the hereditary disorders that prevail in the family to be carried into the baby. The preimplantation techniques involve various steps like the collection of eggs from the mother or egg donor which are later in vitro fertilized. Fertilized eggs are then tested for various genetic conditions through screening processes.
Healthy embryos may be frozen and stored for further use whereas unfit embryos are destroyed. The healthy embryos are implanted to induce pregnancy. Preimplantation genetic testing also serves another purpose like gender selection.
However, this application is currently facing several ethical questions. The preimplantation genetic testing is currently is gaining popularity as a fertility treatment option among carriers of sex-linked genetic disorders, single gene donors, people suffering from chromosomal disorders, older women seeking pregnancy and among women who experience recurring abortions.
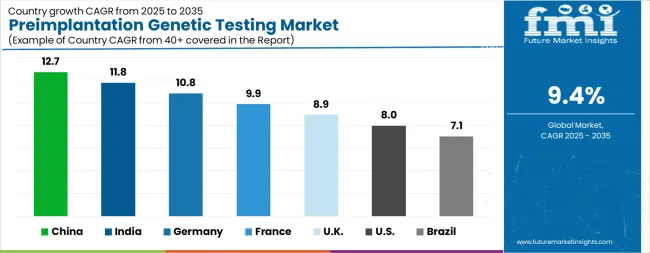
| Country | CAGR |
|---|---|
| China | 12.7% |
| India | 11.8% |
| Germany | 10.8% |
| France | 9.9% |
| UK | 8.9% |
| USA | 8.0% |
| Brazil | 7.1% |
The Preimplantation Genetic Testing Market is expected to register a CAGR of 9.4% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 12.7%, followed by India at 11.8%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 7.1%, yet still underscores a broadly positive trajectory for the global Preimplantation Genetic Testing Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 10.8%. The USA Preimplantation Genetic Testing Market is estimated to be valued at USD 345.5 million in 2025 and is anticipated to reach a valuation of USD 745.2 million by 2035. Sales are projected to rise at a CAGR of 8.0% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 50.5 million and USD 30.8 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 934.5 Million |
| Application Type | Aneuploidy Screening, Gender Screening, Chromosomal Aberration Screening, HLA Typing, Single Gene Disorder Screening, and Others |
| Product Type | Reagents, Instruments, Analyzer Software, and Accessories & Consumables |
| End User | Fertility Clinics & Maternity Centers, Hospitals, Diagnostic Laboratories, and Academic Institutions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
| Key Companies Profiled | Agilent Technologies, Fulgent Genetics, Genea BIOMEDX, Illumina, Medicover Genetics, Natera, Revvity, RGI, Takara Bio, Thermo Fisher Scientific, Varinos, Virtus Health, and Vitrolife Group (Igenomix) |
The global preimplantation genetic testing market is estimated to be valued at USD 934.5 million in 2025.
The market size for the preimplantation genetic testing market is projected to reach USD 2,294.8 million by 2035.
The preimplantation genetic testing market is expected to grow at a 9.4% CAGR between 2025 and 2035.
The key product types in preimplantation genetic testing market are aneuploidy screening, gender screening, chromosomal aberration screening, hla typing, single gene disorder screening and others.
In terms of product type, reagents segment to command 33.4% share in the preimplantation genetic testing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Genetic Analyzers Market Size and Share Forecast Outlook 2025 to 2035
Genetically Modified Food Market Analysis by Type, Trait, and Region through 2035
Genetic Cardiomyopathies Market
Genetic Leukemia Detection Testing Market
Cytogenetic Systems Market Size and Share Forecast Outlook 2025 to 2035
Animal Genetics Market Size and Share Forecast Outlook 2025 to 2035
Bone Morphogenetic Protein Market Size and Share Forecast Outlook 2025 to 2035
Cellular Epigenetics Market
Molecular Cytogenetics Market Size and Share Forecast Outlook 2025 to 2035
Pre-Pregnancy Genetic Testing Market Size and Share Forecast Outlook 2025 to 2035
Point-Of-Care Genetic Testing Market
Cancer-focused Genetic Testing Service Market Analysis – Growth & Industry Insights 2024-2034
Companion Animal Genetics Market Size and Share Forecast Outlook 2025 to 2035
Direct-to-Consumer Genetic Testing Market Analysis - Trends & Outlook 2025 to 2035
Testing, Inspection & Certification Market Growth – Trends & Forecast 2025 to 2035
5G Testing Market Size and Share Forecast Outlook 2025 to 2035
AB Testing Software Market Size and Share Forecast Outlook 2025 to 2035
5G Testing Equipment Market Analysis - Size, Growth, and Forecast 2025 to 2035
Eye Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HSV Testing Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA