The surgical tourniquet market is gaining consistent momentum, supported by the increasing volume of orthopedic and trauma surgeries worldwide and the growing demand for bloodless surgical fields. Medical device press releases and orthopedic association reports have emphasized the expanding use of tourniquet systems in procedures requiring precise visualization, particularly in joint replacements and reconstructive surgeries.
Hospitals and surgical centers have been prioritizing efficiency and intraoperative safety, contributing to wider adoption of pneumatic and electronic tourniquet systems. Additionally, the development of tourniquets with integrated safety features such as pressure regulation, alarms, and data tracking has enhanced surgeon confidence and patient outcomes.
The aging population, rising prevalence of osteoarthritis, and increasing incidence of sports-related injuries have also elevated the demand for tourniquet-assisted procedures. Looking forward, continued innovation in cuff materials, pressure sensors, and infection-resistant designs are expected to drive market advancement. The segmental leadership of tourniquet systems, knee arthroplasty procedures, and hospital settings reflects clinical preferences for controlled hemostasis, advanced system reliability, and institutional procurement policies.

| Metric | Value |
|---|---|
| Surgical Tourniquet Market Estimated Value in (2025 E) | USD 573.4 million |
| Surgical Tourniquet Market Forecast Value in (2035 F) | USD 952.0 million |
| Forecast CAGR (2025 to 2035) | 5.2% |
The market is segmented by Product, Application, and End-User and region. By Product, the market is divided into Tourniquet Systems and Tourniquet Cuffs. In terms of Application, the market is classified into Knee Arthroplasty, Amputation of Limbs, Plastic Surgeries, Trauma Cases, and Joint Replacement. Based on End-User, the market is segmented into Hospitals, Ambulatory Surgical Centers, and Specialized Clinics. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
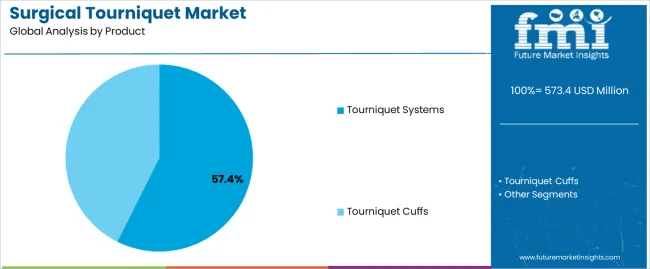
The Tourniquet Systems segment is projected to generate 57.4% of the surgical tourniquet market revenue in 2025, sustaining its lead over other product categories. This segment’s growth has been influenced by the rising need for precise, controllable, and safe hemostasis during surgeries.
Hospitals and surgical teams have preferred advanced tourniquet systems for their programmable pressure settings, dual cuff management, and integrated safety alarms, which help minimize the risk of nerve damage and tissue injury.
Furthermore, modern tourniquet systems have been designed for better data recording and real-time monitoring, aligning with hospital protocols on surgical performance and patient safety. The growing focus on operating room efficiency and compliance with surgical guidelines has increased the use of these systems across orthopedic, vascular, and trauma procedures. As minimally invasive surgeries gain popularity, tourniquet systems offering superior control and quicker application have gained traction, reinforcing their dominant share in the market.
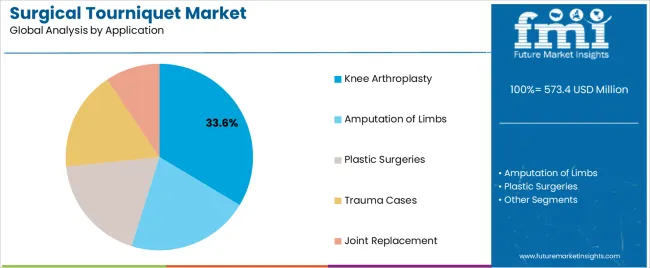
The Knee Arthroplasty segment is anticipated to hold 33.6% of the surgical tourniquet market revenue in 2025, making it the leading application area. Growth of this segment has been propelled by the global rise in total and partial knee replacement surgeries, particularly among aging populations.
Clinical guidelines and orthopedic publications have reported that the use of surgical tourniquets during knee arthroplasty contributes to improved operative visibility and reduced blood loss, enabling more accurate prosthesis placement.
Hospitals have standardized tourniquet usage for knee procedures due to its benefits in maintaining a clear surgical field and minimizing operative time. Additionally, tourniquet application has been favored in both traditional and minimally invasive knee arthroplasties, expanding its relevance across surgical techniques. With the growing incidence of osteoarthritis and the increasing adoption of fast-track orthopedic surgery protocols, the Knee Arthroplasty segment is expected to maintain strong demand for tourniquet systems in surgical planning and execution.
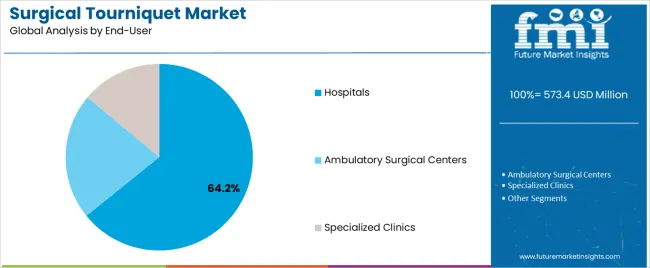
The Hospitals segment is projected to contribute 64.2% of the surgical tourniquet market revenue in 2025, preserving its dominance in end-user adoption. This growth has been driven by the high surgical volumes, infrastructure capabilities, and skilled workforce found in hospital settings.
Hospitals have served as the primary point of care for complex orthopedic and trauma surgeries, where tourniquet usage is integrated into standard operating procedures. Medical device procurement teams have favored tourniquet systems with advanced monitoring features, ensuring compliance with surgical safety protocols and reducing postoperative complications.
Moreover, hospitals have benefited from centralized sterilization, maintenance, and staff training programs, which have enabled consistent usage of both reusable and single-use tourniquet systems. Clinical audits and performance reviews conducted within hospitals have underscored the role of tourniquets in enhancing procedural outcomes, further encouraging their institutional adoption. As hospitals continue to invest in surgical technologies and expand orthopedic units, this segment is expected to remain the cornerstone of surgical tourniquet demand.
The table presents the projected CAGRs for several semi-annual periods between 2025 and 2035 for the surgical tourniquet market. The growth rate of the business is expected to reach 5.9% in the first half (H1) of the decade of 2025 to 2035, followed by a slightly lower 5.6% in the second half (H2).
| Particular | Value CAGR |
|---|---|
| H1 | 5.9% (2025 to 2035) |
| H2 | 5.6% (2025 to 2035) |
| H1 | 5.5% (2025 to 2035) |
| H2 | 5.1% (2025 to 2035) |
Throughout the subsequent period, between H1 2025 and H2 2035, the CAGR is expected to decrease slightly to 5.5% in the first half and hold 5.1% in the second half.
Modern Technologies and Regulatory Approvals to Drive Growth
A number of public health initiatives have emphasized the importance of controlling bleeding during accidents and trauma in order to reduce mortality and morbidity. As obesity and its associated bariatric surgery prevalence increases, tourniquets are being used to manage blood flow during bariatric surgeries.
In addition to government funding and the development of healthcare infrastructures, the industry is also benefitting from the expansion of healthcare infrastructure.
In clinical settings, tourniquets are approved by regulatory agencies and certified for effectiveness and safety. As tourniquets become more accepted and approved, the industry is expected to grow. The introduction of modern technologies, such as Bluetooth-enabled tourniquets, can also be credited with making the industry more advanced.
Increasing demand for patient-specific tourniquet solutions, which are tailored to individual requirements and needs, is increasing due to their success in improving outcomes.
An increase in postoperative rehabilitation outcomes is associated with the use of tourniquets, particularly in orthopedic and limb surgery. As a result of these positive correlations, their use is increased.
Advanced Trauma Care to Provide Lucrative Opportunities
In recent years, advances in biomedical engineering have improved the ability to customize tourniquet systems according to surgeons and procedures, improving safety and efficiency.
A number of advancements in tourniquet technology have produced improved cuffs capable of creating a safe, low-pressure gradient on the underlying patient limbs, as well as systems capable of applying pressure automatically for a short period of time.
The popularity of aesthetic procedures and best-in-class trauma care drive demand in the market. Through the use of surgery tourniquets, surgeons can minimize perioperative blood loss and optimize antibiotic delivery.
A number of new tourniquet inflation devices, such as S-MART, aim to improve patient safety and reduce complications associated with tourniquet use. Trials and studies with a larger sample size will be conducted in order to prove the efficacy of these products.
Surgical Tourniquets Demand Side Trends
The healthcare industry is evolving, directed by catalysts like population growth, aging population, and innovation in medical technology. Within the industry, the demand for surgical tourniquets upswing as surgical processes become pervasive.
Adherence to regulatory standards is essential in the medical device sector. The vendors should be informed about shifts in rules and standards to warrant that products satisfy the essential needs of new entrants.
Training medical care professionals regarding the advantages and proper adoption of operative tourniquets is vital. The manufacturers can create learning materials or training projects to ensure that users comprehend the best activities and health precautions.
Tourniquet adoption during extremities surgery became widespread in orthopedics, as per a paper published in July 2025 in the Journal of Experimental Orthopedics. Around 90% of surgeons in the United States and the United Kingdom are thought to use tourniquets when doing total knee arthroplasty regularly.
Factors Adversely Affecting Surgical Tourniquet Industry Size Expansion
Economic fluctuations in medical care expenditure impede the growth for the market. The industry players should be prepared to traverse through periods of economic unreliability and modify their strategies correspondingly.
Adherence to strict regulatory standards and shifting guidelines are challenging for vendors functioning in the sector. Compliance with the regulations needs considerable investments in research, development, and quality control procedures.
As with any medical equipment, there are inherent threats related to the implementation of tourniquets in surgeries. The producers invest in extensive liability insurance and administer rigorous quality assurance standards to curb the hazard of product-related occurrences and legal actions.
The global surgical tourniquet market reached USD 445.1 million in 2020. Subsequently, demand for surgical tourniquets exhibited an HCAGR of 6.2% and acquired USD 573.4 million in 2025.
The industry has witnessed notable growth from 2020 to 2025, ushered by factors like a spur in surgical processes, improvement in medical technology, and surging consciousness regarding patient safety. This period experienced an upsurge in demand, with prominent vendors launching novel products to satisfy the developing requirements of healthcare providers.
Between 2025 and 2035, the demand for surgical tourniquets is on an upward trajectory. Catalysts aiding this growth are the pervasiveness of chronic disorders compelling surgical interventions, proliferating healthcare institutions in developing countries, and an aging population needing surgical interventions.
Developments in surgical procedures and a pressing focus on patient safety augment the adoption of these devices. The soaring investments in healthcare research and development and the launch of advanced tourniquet systems stimulate the growth during the forecast period.
The industry is slated for considerable growth between 2025 and 2035, proffering lucrative opportunities for providers to cash on the evolving demand.
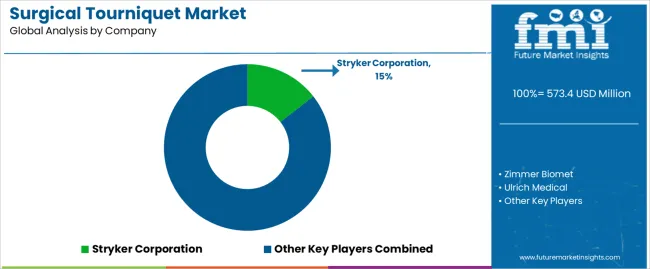
A significant 28.7% share of the global market has been captured by companies in the Tier 1 category. Tourniquets in surgical procedures are part of the product offerings of these well-established medical device companies. With their strong financial positions, distribution channels, and research and development resources, they have an edge in the market.
Their innovative capabilities and ability to meet the diverse needs of customers around the world indicate that they will remain at the forefront. Prominent players include Stryker Corporation and Zimmer Biomet.
Tier 2 companies represent a 34.2% share of the industry is higher than that of tier 1 companies, demonstrating a strong presence overseas and an in-depth understanding of the industry. Surgical tourniquet companies in Tier 2 are specialized in this area.
Quality and innovation must remain at the forefront of this niche sectors. While niche players command a relatively small share of the market, they have a larger reach and financial clout than Tier 1 players. Their specialization and targeted approach enable them to deliver excellent results. Prominent tier 2 players include VBM Medizintechnik and Delfi Medical Innovations.
Most small firms in Tier 3 are regionally dominant and specialize in a narrow range of products. Offering a broad range of surgical products that are tailored to specific customer requirements in order to offer cost-effective solutions.
Players will perform exponentially in the industry if they comply with international standards and stringent regulations. Players include Ulrich Medical, Anetic Aid, Hammarplast Medical, Daesung Maref, OHK Medical Devices, and Dessillons & Dutrillaux.
The section below covers the forecast for emerging markets for surgical tourniquets in terms of countries. Information on key countries in several parts of the globe, including North America, Asia Pacific, Europe, and others, is provided.
The United States is anticipated to remain at the forefront in North America, with a CAGR of 5.8% through 2035. In Asia Pacific region, India experiences a CAGR of 7.7% by 2035.
| Countries | CAGR 2025 to 2035 |
|---|---|
| United States | 5.8% |
| Germany | 6.2% |
| United Kingdom | 4.3% |
| India | 7.7% |
| China | 7.9% |
The United States is encouraged by modern medical care facilities and a strong demand for innovative healthcare devices. The flourishing consciousness concerning the advantages of minimally invasive surgical methods assists in the growth of the market.
The role of regulatory bodies in governing the surgical tourniquet sector market ushers the adoption of tourniquet technologies in the United States.
The pervasiveness of chronic illnesses and the inclining geriatric population ushered in the demand for the market in the United States. Technological innovations and partnerships among essential vendors advance the industry expansion in the United States.
The market in Germany thrives on a focus on accuracy and quality, indicating the impact of new technologies like artificial intelligence on the market. The strict rules and emphasis on product efficiency direct innovation and expansion in Germany's medical tourniquet market.
Germany experiences growth amplified by a strengthening number of surgeries and proliferating investments in healthcare facilities. The commitment to patient safety and high standards of healthcare delivery in Germany stimulate the growth avenue of the market.
The market in India witnessed steady growth characterized by a proliferating healthcare system and widening medical tourism. The adoption of digital health tech and telemedicine platforms augments the business in India, particularly in remote regions with constrained access to medical care amenities.
The disposable and environmental sustainability concerns catapult the use of the instruments in India. The towering investments in healthcare units and government schemes to encourage medical device production bolster the growth in the market.
The section contains information about the leading segments in the surgical tourniquet industry. In terms of product type category, the tourniquet cuffs segment garners a share of 78.4% by 2035. By application category, the knee arthroplasty segment takes precedence with a share of 33.1% in 2035.
| Segment | Tourniquet Cuffs |
|---|---|
| Value Share (2035) | 78.4% |
Tourniquet cuffs are usually cheaper as compared to complete tourniquet systems, making them widely available and easier to purchase. They are replaced frequently because of wear and hygiene rules, spurring significant sales.
Hospitals and surgical centers prioritize storing tourniquet cuffs because of their vital role and standard demand in surgeries. Cuffs are used with diverse tourniquet systems, improving their flexibility and demand. As the number of surgeries escalates, the demand for tourniquet cuffs also rises, resulting in a steady boom in sales.
| Segment | Joint Replacement |
|---|---|
| Value Share (2035) | 33.1% |
A tourniquet is a widely accepted and effective practice during knee replacement surgery. In addition to preventing blood loss, it improves the visibility of the wound and stops the bleeding. The use of tourniquets during joint arthroplasty surgery further prevents bleeding from resurfaced bones, so bone-implant cementation can proceed more successfully.
Tourniquets may improve the long-term survival of knee implants by reducing bleeding from porous bone ends, thereby improving the bond between the soft cement and the bone. The tourniquet can reduce the frequency of suctioning and reduce complication risk during surgery.
Key surgical tourniquet vendors use promotional and distribution procedures, like direct sales to medical care experts and sales to public health facilities. To keep up with demand globally, surgical tourniquet providers are strengthening their manufacturing capacity.
The majority of the leading producers are not only meeting the demand but also innovating. They are focusing on locating and making advantageous technologies and targeted products from internal as well as external sources.
This active approach is targeted at satisfying the unsatisfied demand in emerging economies, sustaining a positive sense of the industry's future. The manufacturers adopt partnerships and acquisitions as their primary strategies to intensify sales across regions.
Investment Analysis of Specific Surgical Tourniquet Companies
| Company | Stryker Corporation |
|---|---|
| Headquarter | United States |
| Recent Advancement | In February 2025, Stryker announced the acquisition of Vocera Communications, Inc. which is a pioneer in digital care collaboration and communication. |
| Company | Zimmer Biomet |
|---|---|
| Headquarter | United States |
| Recent Advancement | Zimmer Biomet Holdings, Inc. announced the acquisition of Embody, Inc. in January 2025, which is focused on soft tissue healing, for USD 155 million at closing and an additional USD 120 million determined to achieve regulatory and commercial milestones over three years. The acquisition is cumulative to the revenue growth and moderately dilutive to earnings per share. |
| Company | Ulrich GmbH & Co.KG |
|---|---|
| Headquarter | Germany |
| Recent Advancement | Bracco and Ulrich GmbH Co KG, a popular medical device producer specializing in media injectors and spinal implants, announced a long-term partnership in November 2025 that brings a Bracco-branded modern MR injector to the United States. |
The industry is bifurcated into tourniquet systems and tourniquet cuffs. The tourniquet systems category is further bifurcated into single channel systems and dual channel systems.
The tourniquet cuffs category is further bifurcated into inflatable cuffs and non-inflatable cuffs. The inflatable cuffs sub-segment in further bifurcated into disposable cuffs and reusable cuffs. The non-inflatable cuffs sub-segment in further bifurcated into disposable cuffs and reusable cuffs
The application category is classified into knee arthroplasty, amputation of limbs, plastic surgeries, and trauma cases.
The end user category is trifurcated into hospitals, ambulatory surgical centers, and specialized clinics.
The global surgical tourniquet market is estimated to be valued at USD 573.4 million in 2025.
The market size for the surgical tourniquet market is projected to reach USD 952.0 million by 2035.
The surgical tourniquet market is expected to grow at a 5.2% CAGR between 2025 and 2035.
The key product types in surgical tourniquet market are tourniquet systems, _single channel systems, _dual channel systems, tourniquet cuffs, _inflatable cuffs and _non-inflatable cuffs.
In terms of application, knee arthroplasty segment to command 33.6% share in the surgical tourniquet market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Surgical Operating Microscope Market Forecast and Outlook 2025 to 2035
Surgical Heart Valves Market Size and Share Forecast Outlook 2025 to 2035
Surgical Aspirators Market Size and Share Forecast Outlook 2025 to 2035
Surgical Robot Procedures Market Size and Share Forecast Outlook 2025 to 2035
Surgical Wound Care Market Size and Share Forecast Outlook 2025 to 2035
Surgical Retractors Market Size and Share Forecast Outlook 2025 to 2035
Surgical Drainage Devices Market Size and Share Forecast Outlook 2025 to 2035
Surgical Booms Market Insights - Size, Share & Industry Growth 2025 to 2035
Surgical Scissors Market Size and Share Forecast Outlook 2025 to 2035
Surgical Instruments Tracking System Market Growth - Trends & Forecast 2025 to 2035
Surgical Instruments Packaging Market Size, Share & Forecast 2025 to 2035
Surgical Monitors Market Analysis - Industry Insights & Forecast 2025 to 2035
Surgical Scalpels Market Trends – Growth & Forecast 2025-2035
Surgical Generators Market – Trends & Forecast 2025 to 2035
Surgical Gloves Market Trends - Size, Demand & Forecast 2025 to 2035
Surgical Clips Market Analysis - Size, Share & Forecast 2025 to 2035
Surgical Mask Market Insights - Growth & Forecast 2025 to 2035
Surgical Drapes Market Overview - Growth, Demand & Forecast 2025 to 2035
Surgical Stapling Device Market is segmented by product, Usage Type, Stapling Type, Indication and End User from 2025 to 2035
Key Companies & Market Share in the Surgical Scrub Sector

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA