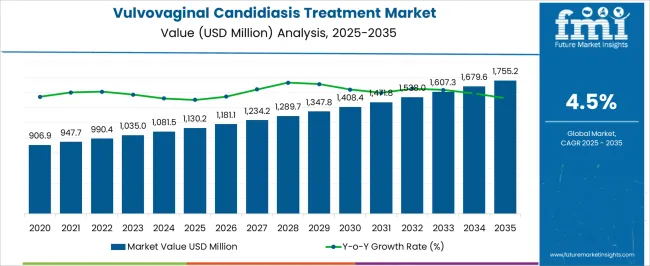
The Vulvovaginal Candidiasis Treatment Market is estimated to be valued at USD 1130.2 million in 2025 and is projected to reach USD 1755.2 million by 2035, registering a compound annual growth rate (CAGR) of 4.5% over the forecast period.
| Metric | Value |
|---|---|
| Vulvovaginal Candidiasis Treatment Market Estimated Value in (2025 E) | USD 1130.2 million |
| Vulvovaginal Candidiasis Treatment Market Forecast Value in (2035 F) | USD 1755.2 million |
| Forecast CAGR (2025 to 2035) | 4.5% |
The vulvovaginal candidiasis treatment market is progressing steadily due to increasing infection prevalence, higher recurrence rates, and greater awareness among women about early diagnosis and treatment options. A rise in risk factors such as diabetes, antibiotic usage, hormonal fluctuations, and immune suppression has contributed to consistent demand for effective antifungal therapeutics.
Advances in drug formulation, along with patient education initiatives led by healthcare providers, are further improving diagnosis and treatment uptake. Healthcare systems are emphasizing fast acting, broad spectrum antifungal therapies that ensure reduced recurrence and ease of administration.
Future growth will be supported by ongoing development of novel azole and non azole treatments, greater access to over the counter medications, and improved healthcare infrastructure in emerging regions. The market remains favorable as patient centric treatment protocols and enhanced pharmaceutical distribution networks continue to evolve.
The market is segmented by Drug Class, Route of Administration, and Distribution Channel and region. By Drug Class, the market is divided into Clotrimazole, Nystatin, Fluconazole, Ketoconazole, Terbinafine, Terconazole, and Others. In terms of Route of Administration, the market is classified into Oral, Intravenous, and Topical. Based on Distribution Channel, the market is segmented into Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
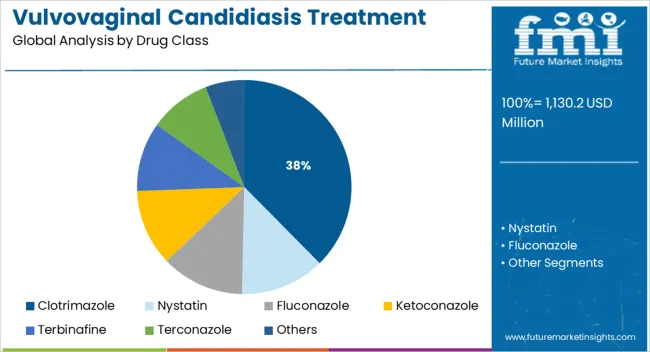
The clotrimazole segment is anticipated to account for 37.60% of total revenue within the drug class category by 2025, emerging as the leading treatment option. Its dominance is attributed to its broad spectrum antifungal activity, proven efficacy in both acute and recurrent infections, and availability in various formulations including oral, topical, and vaginal routes.
Clotrimazole’s affordability and long standing inclusion in clinical guidelines have reinforced its widespread prescription across both developed and developing healthcare settings. Patient preference for familiar and fast acting treatment options has further sustained demand.
As clinicians continue to favor well tolerated and cost effective therapies, clotrimazole remains a cornerstone in the treatment of vulvovaginal candidiasis.
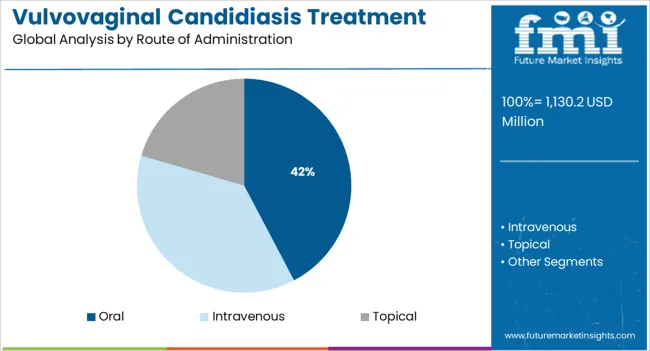
The oral administration segment is projected to contribute 42.30% of total revenue by 2025 within the route of administration category, positioning it as the most utilized method. This trend is driven by patient preference for ease of use, improved adherence, and systemic action, especially in cases of recurrent or severe infections.
Oral therapies offer the convenience of single dose regimens and eliminate the need for local application, which can be challenging for certain patient populations. Furthermore, the availability of oral antifungals in both prescription and over the counter formats has increased their accessibility.
As treatment protocols continue to emphasize convenience and systemic efficacy, the oral route remains the preferred administration pathway in managing vulvovaginal candidiasis.
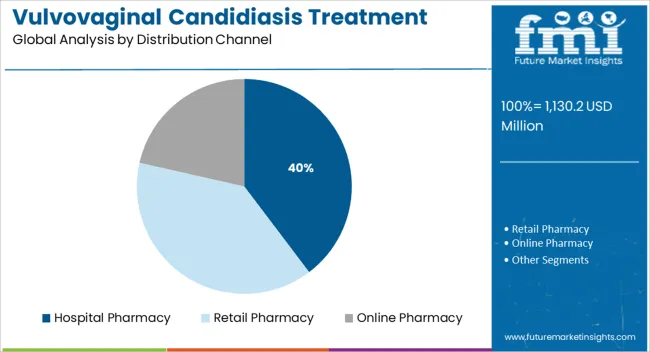
The hospital pharmacy segment is expected to hold 39.70% of the total market revenue by 2025 within the distribution channel category, making it the leading channel. This is primarily due to the high rate of diagnosis and treatment initiation occurring in clinical settings where access to hospital pharmacies is immediate.
Hospital pharmacies maintain a comprehensive stock of antifungal medications, including both generic and branded formulations, and are equipped to provide treatment for both initial and recurrent infections. Additionally, the assurance of clinical oversight during prescription and dispensing enhances patient confidence and compliance.
With hospitals serving as the first point of care for many symptomatic patients, especially in urban centers, this channel continues to dominate distribution in the vulvovaginal candidiasis treatment market.
According to market research and competitive intelligence provider FMI, the vulvovaginal candidiasis industry grew at a CAGR of 4.3% from 2020 to 2025.
Vaginal yeast infection is a common fungal infection and is called candidal vaginitis or candidal vulvovaginitis Symptoms of vaginal yeast infection include itching, burning sensation or pain during urination or sexual intercourse, swelling, rashes, and redness.
Candida albicans are the most prevalent cause of fungal infections in people. Candida species are the most common cause of fungal urinary tract infections (UTIs). Candida UTIs can occur in the lower portion of the urinary tract or in some cases can ascend up to the kidneys.
The antifungal drug fluconazole can be used in many cases. VVC prevalence has increased due to growing diabetes cases and repeated antibiotic usage among individuals.
The market is expected to grow due to an increase in Research and Development initiatives for precise diagnosis. Various government and non-government bodies, including the World Health Organization and the European Institute of Women's Health (EIWH), have launched public awareness campaigns about women's health.
Demand for the VVC treatment market is expected to expand at a CAGR of 4.5% from 2025 to 2035, with the global market predicted to reach a valuation of USD 1755.2 Million by 2035.
The growing prevalence of vulvovaginal candidiasis infection is further driving the demand for new therapeutics for treatment, augmenting the growth of the market
The increasing prevalence of vulvovaginal candidiasis infection is the primary factor driving the market growth. For instance, vulvovaginal candidiasis affects about 138 million women annually (range 103 to 172 million), with a global annual prevalence of 3871 per 100 000 women; 372 million women are affected by recurrent vulvovaginal candidiasis over their lifetime. In addition, recurrent VVC prevalence is rising, affecting more than 9% of women annually.
The high growth rate can be attributed to the rising disease burden of VVC and increasing testing rates. Positive changes, such as healthcare benefits by the government, increased awareness among consumers, and willingness to avail medical treatments are also expected to drive the growth of the vulvovaginal candidiasis treatment market.
Also, an increase in healthcare spending and the growth of healthcare infrastructure are also driving the global acute vulvovaginal candidiasis treatment market.
Increased Incidence of Vaginal Infections and Rising Research and development activities & Public Awareness about Women's Health
Vaginal yeast infection is a common fungal infection and is called candidal vaginitis or candidal vulvovaginitis (CVV)
An increase in the global burden of various ailments such as cardiovascular, neurological, and gastrointestinal diseases as well as cancer has boosted the consumption of broad-spectrum antibiotics, thereby propelling the number of yeast infection cases.
The rapid increase in the global disease burden, coupled with escalating demand for better treatment options and an increasing number of hospital-acquired infections, is also propelling the demand for bacterial vulvovaginal candidiasis treatment.
Furthermore, expanding healthcare infrastructure in developing economies, availability of pharmaceutical drugs, and robust technological advancements in the biopharmaceutical industries are all contributing to significant opportunities for vulvovaginal candidiasis treatment over the forecasted period.
Lack of Awareness of Vulvovaginal Candidiasis Treatment
Although the vulvovaginal candidiasis treatment market has numerous end-uses, there are some obstacles that likely pose a challenge to market growth during the forecasting.
The lack of awareness among people in developing countries may restrain the market growth. Also, the lack of experience in using advanced technology and the high cost associated with the diagnosis and treatment procedure is expected to act as a restraint to the market.
Developed Healthcare Facilities, High Incidence of Diseases, and Presence of Key Producers in the North America Market
The North American vulvovaginal candidiasis treatment market is expected to account for the highest market share of around 39.2% in the global landscape in 2025.
The region is expected to expand further at a steady growth rate maintaining its dominant position throughout the forecast period. This dominance can be attributed to the rise in patient awareness and increased healthcare expenditure in the region.
A recent Harvard study showed that there are approximately 6 Million American women who are suffering from vulvodynia. This region's established healthcare infrastructures, as well as the high frequency of disease and the presence of important manufacturers, are major factors contributing towards vulvovaginal candidiasis treatment market share.
Product approvals and launches for treatment of vulvovaginal candidiasis treatment to boost the growth of the vulvovaginal candidiasis treatment market in North America.
For instance, In June 2024, Scynexis, Inc. announced the FDA approval for their novel drug BREXAFEMME indicated for VVC treatment. All these launches are anticipated to fuel the industry's growth in the region.
Well-established Healthcare Infra in Europe Driving Vulvovaginal Candidiasis Treatment
Europe is one of the top regions driving vulvovaginal candidiasis treatment demand. The country's prospects are being boosted by the presence of a well-developed healthcare infrastructure and the availability of skilled medical staff.
Advanced therapeutic options, new approvals, launches, and proactive government measures further contribute to regional vulvovaginal candidiasis treatment market growth.
Throughout the projected period, Europe’s market is expected to grow at a robust rate. The country is boosting its spending on biopharmaceutical research and development in order to develop cures for uncommon diseases, which bodes well for vulvovaginal candidiasis treatment system providers in the region. The market in Europe is expanding rapidly, due to an increase in the patient population, especially in the United Kingdom and Germany.
Rising Awareness of Vaginal Disorders & Availability of Advanced Treatment Aiding Sales of Vulvovaginal Candidiasis Treatment Market
During the projection period, the Asia Pacific vulvovaginal candidiasis treatment market is predicted to develop at the fastest rate. This is due to a significant number of unmet clinical requirements, improved knowledge of early diagnosis, and the availability of effective medications.
Developing economies such as China and Japan are projected to contribute to the expansion of the market in the Asia Pacific due to better healthcare infrastructure, economic growth, an increase in the number of insurance payers, an expansion of the private healthcare sector, and an increase in education and awareness.
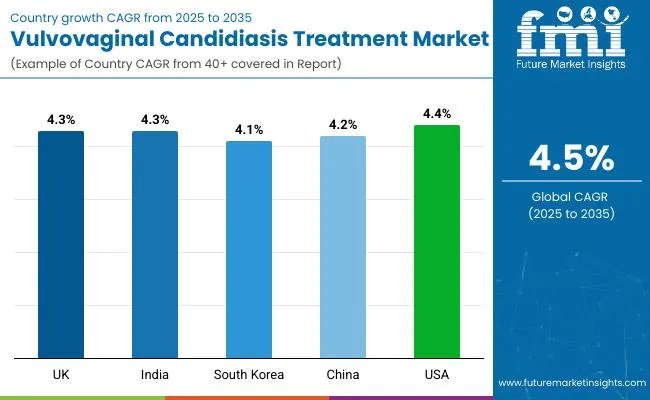
| Attributes | Details |
|---|---|
| United Kingdom | 4.3% |
| India | 4.3% |
| South Korea | 4.1% |
| China | 4.2 % |
| USA | 4.4 % |
Viamet Pharmaceuticals, and Cidara Therapeutics, are some of the start-ups in the vulvovaginal candidiasis treatment market
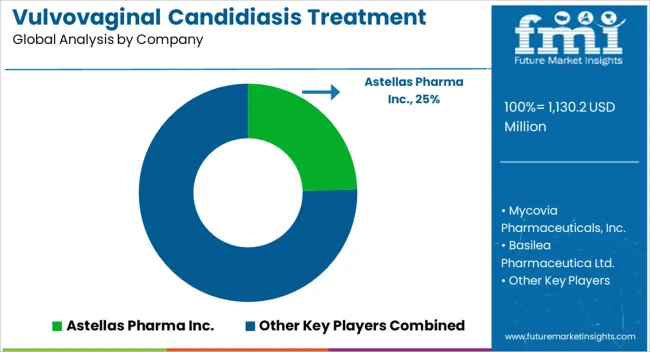
Astellas Pharma Inc., Mycovia Pharmaceuticals, Inc., Basilea Pharmaceutica Ltd., Scynexis, Inc., Grupo Ferrer Internacional S.A., Pfizer, Inc., Cadila Pharmaceuticals, Bayer AG., Bristol-Myers Squibb Company are some of the key companies in the vulvovaginal candidiasis treatment market.
These players are continuously adopting various strategies such as new product launches, facility expansions, mergers, collaborations, partnerships, and acquisitions to increase their revenue share and gain a competitive edge in the market. for instance,
In October 2025 - Astellas Pharma Inc. and Pantherna Therapeutics GmbH announced that the companies have entered into a new technology evaluation agreement for research to generate mRNA-based regenerative medicine programs using direct reprogramming (transdifferentiation)*.
In August 2025 – Mycovia Pharmaceuticals, Inc. announced that it will present VIVJOA™ (oteseconazole) capsules in patients with recurrent vulvovaginal candidiasis (RVVC) at the 2025 Infectious Diseases Society for Obstetrics and Gynecology (IDSOG) Annual Meeting. VIVJOA is the first and only FDA-approved medication for RVVC indicated to reduce the incidence of RVVC in females with a history of RVVC who are NOT of reproductive potential
In July 2025 – Mycovia Pharmaceuticals, Inc. announced the USA availability of VIVJOA™ (oteseconazole) capsules, an azole antifungal indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are NOT of reproductive potential.
In September 2024 - Basilea Pharmaceutica Ltd. and its partner Asahi Kasei Pharma Corporation filed a New Drug Application (NDA) for the marketing authorization of isavuconazole in Japan for the treatment of the fungal infections aspergillosis, mucormycosis and cryptococcosis.
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR 4.5% from 2025 to 2035 |
| Expected Market Value (2025) | USD 1130.2 million |
| ProjectedForecast Value (2035) | USD 1755.2 million |
| Base Year for Estimation | 2025 |
| Historical Data | 2020 to 2025 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in USD Million and CAGR 4.5% from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends, and Pricing Analysis |
| Segments Covered | Drug Class, Route of administration, Distribution Channel, Regions |
| Regions Covered | North America; Latin America; Europe; Asia Pacific; Middle East and Africa(MEA) |
| Key Countries Profiled | USA, Canada, Brazil, Mexico, Germany, United Kingdom, France, Spain, Italy, Malaysia, Thailand, India, Singapore, GCC Countries, South Africa, Israel |
| Key Companies Profiled | Astellas Pharma Inc.; Mycovia Pharmaceuticals, Inc.; Basilea Pharmaceutica Ltd.; Scynexis, Inc.; Grupo Ferrer Internacional S.A.; Pfizer, Inc.; Cadila Pharmaceuticals; Bayer AG.; Bristol-Myers Squibb Company |
| Customization | Available Upon Request |
The global vulvovaginal candidiasis treatment market is estimated to be valued at USD 1,130.2 million in 2025.
The market size for the vulvovaginal candidiasis treatment market is projected to reach USD 1,755.2 million by 2035.
The vulvovaginal candidiasis treatment market is expected to grow at a 4.5% CAGR between 2025 and 2035.
The key product types in vulvovaginal candidiasis treatment market are clotrimazole, nystatin, fluconazole, ketoconazole, terbinafine, terconazole and others.
In terms of route of administration, oral segment to command 42.3% share in the vulvovaginal candidiasis treatment market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Candidiasis Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market
Water Treatment System Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Chemical Market Size and Share Forecast Outlook 2025 to 2035
Algae Treatment Chemical Market Forecast and Outlook 2025 to 2035
Water Treatment Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Equipment Market Size and Share Forecast Outlook 2025 to 2035
Burns Treatment Market Overview – Growth, Demand & Forecast 2025 to 2035
CRBSI Treatment Market Insights - Growth, Trends & Forecast 2025 to 2035
Water Treatment Polymers Market Growth & Demand 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA