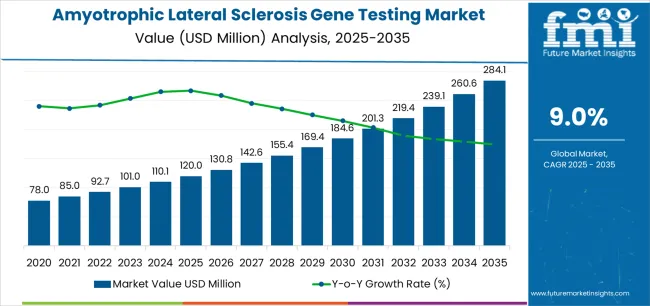
The amyotrophic lateral sclerosis gene testing market is valued at USD 120 million in 2025 and is slated to reach USD 284 million by 2035, recording an absolute increase of USD 164 million over the forecast period. This translates into a total growth of 136.7%, with the market forecast to expand at a compound annual growth rate (CAGR) of 9% between 2025 and 2035. The overall market size is expected to grow by approximately 2.37X during the same period, supported by increasing awareness of ALS genetic factors, growing adoption of precision diagnostic technologies across neuromuscular and genetic testing sectors, and rising preference for personalized medicine solutions in clinical trial applications.
The amyotrophic lateral sclerosis gene testing market represents a specialized segment of the global genetic diagnostics industry, characterized by clinical innovation and robust demand across neuromuscular centers, reference laboratories, and family screening channels. Market dynamics are influenced by evolving diagnostic paradigms toward genetic profiling, growing interest in targeted therapeutic development, and expanding partnerships between genetic testing companies and pharmaceutical sponsors in developed and emerging economies. Traditional clinical diagnosis approaches continue evolving as clinicians seek proven genetic alternatives that offer enhanced diagnostic accuracy and reliable prognostic characteristics.
Clinical behavior in the amyotrophic lateral sclerosis gene testing market reflects broader healthcare trends toward precision medicine systems that provide both diagnostic effectiveness benefits and extended patient management improvements. The market benefits from the growing popularity of targeted ALS gene panel applications, which are recognized for their superior diagnostic yield and clinical compatibility across neuromuscular center and reference laboratory applications. Additionally, the versatility of ALS gene testing as both diagnostic tools and research components supports demand across multiple healthcare applications and research segments.
Regional adoption patterns vary significantly, with North American markets showing strong preference for neuromuscular center implementations, while European markets demonstrate increasing adoption of reference laboratory applications alongside conventional clinical systems. The healthcare landscape continues to evolve with sophisticated and targeted diagnostic products gaining traction in mainstream clinical operations, reflecting physician willingness to invest in proven genetic testing technology improvements and precision-oriented features.
The competitive environment features established genetic testing companies alongside specialized ALS diagnostics manufacturers that focus on unique testing capabilities and advanced sequencing methods. Diagnostic accuracy and testing optimization remain critical factors for market participants, particularly as regulatory requirements and clinical standards continue to evolve. Distribution strategies increasingly emphasize multi-channel approaches that combine traditional laboratory supply chains with direct clinical partnerships through genetic counseling agreements and research collaboration contracts.
Market consolidation trends indicate that larger genetic testing companies are acquiring specialty ALS testing developers to diversify their diagnostic portfolios and access specialized sequencing segments. Advanced genomic integration has gained momentum as healthcare companies seek to differentiate their offerings while maintaining competitive diagnostic outcomes. The emergence of specialized testing variants, including enhanced panel formulations and whole genome options, reflects changing clinical priorities and creates new market opportunities for innovative diagnostic system developers.
Amyotrophic Lateral Sclerosis Gene Testing Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value (2025E) | USD 120 million |
| Forecast Value (2035F) | USD 284 million |
| Forecast CAGR (2025 to 2035) | 90% |
Between 2025 and 2030, the amyotrophic lateral sclerosis gene testing market is projected to expand from USD 120 million to USD 185.5 million, resulting in a value increase of USD 65.5 million, which represents 39.9% of the total forecast growth for the decade. This phase of development will be shaped by increasing adoption of targeted gene panel systems, rising demand for neuromuscular center solutions, and growing emphasis on genetic screening features with enhanced diagnostic characteristics. Healthcare facilities are expanding their genetic testing capabilities to address the growing demand for specialized ALS implementations, advanced sequencing options, and patient-specific offerings across clinical segments.
From 2030 to 2035, the market is forecast to grow from USD 185.5 million to USD 284 million, adding another USD 98.5 million, which constitutes 60.1% of the overall ten-year expansion. This period is expected to be characterized by the expansion of clinical trial applications, the integration of innovative sequencing solutions, and the development of specialized testing implementations with enhanced genetic profiles and extended diagnostic capabilities. The growing adoption of advanced genomic formulations will drive demand for ALS gene testing with superior diagnostic characteristics and compatibility with modern genetic technologies across clinical operations.
Between 2020 and 2025, the amyotrophic lateral sclerosis gene testing market experienced robust growth, driven by increasing demand for targeted gene panel systems and growing recognition of genetic testing as essential components for modern ALS diagnosis and research across neuromuscular center and pharmaceutical applications. The market developed as healthcare providers recognized the potential for genetic solutions to provide both diagnostic benefits and operational advantages while enabling streamlined clinical protocols. Technological advancement in sequencing approaches and evidence-based development began emphasizing the critical importance of maintaining diagnostic accuracy and clinical utility in diverse healthcare environments.
Market expansion is being supported by the increasing global awareness of ALS genetic factors and the corresponding need for genetic testing technologies that can provide superior diagnostic benefits and clinical advantages while enabling enhanced patient outcomes and extended compatibility across various neuromuscular and research applications. Modern healthcare providers and genetic specialists are increasingly focused on implementing proven genetic testing technologies that can deliver effective mutation detection, minimize traditional diagnostic limitations, and provide consistent clinical performance throughout complex diagnostic configurations and diverse patient conditions. ALS gene testing proven ability to deliver exceptional diagnostic accuracy against traditional alternatives, enable advanced clinical integration, and support modern precision medicine protocols makes it an essential component for contemporary neuromuscular and research operations.
The growing emphasis on personalized medicine and therapeutic development is driving demand for genetic testing systems that can support complex research requirements, improve patient stratification, and enable advanced clinical trial enrollment. Healthcare preference for technologies that combine effective genetic screening with proven clinical utility and research enhancement benefits is creating opportunities for innovative testing implementations. The rising influence of precision medicine trends and research efficiency awareness is also contributing to increased demand for ALS gene testing that can provide advanced features, seamless clinical integration, and reliable performance across extended research cycles.
The amyotrophic lateral sclerosis gene testing market is poised for robust growth and clinical advancement. As healthcare facilities across North America, Europe, Asia-Pacific, and emerging markets seek technologies that deliver exceptional diagnostic characteristics, advanced genetic capabilities, and reliable clinical options, testing solutions are gaining prominence not just as specialty diagnostics but as strategic enablers of precision medicine technologies and advanced research functionality.
Rising targeted gene panel adoption in neuromuscular center applications and expanding pharmaceutical research initiatives globally amplify demand, while developers are leveraging innovations in sequencing engineering, advanced genetic integration, and diagnostic optimization technologies.
The market is segmented by test type, customer, sample type, delivery model, and region. By test type, the market is divided into targeted ALS gene panel, single-gene, whole exome sequencing, and whole genome sequencing categories. By customer, it covers neuromuscular/ALS centers, reference labs/send-out, at-risk family screening, and clinical trial enrollment/pharma sponsor programs segments. By sample type, it encompasses blood, saliva/buccal swab, and other biofluids segments. By delivery model, it includes hospital integrated lab, commercial reference lab, sponsored/no-cost testing programs, and research/academic core labs categories.
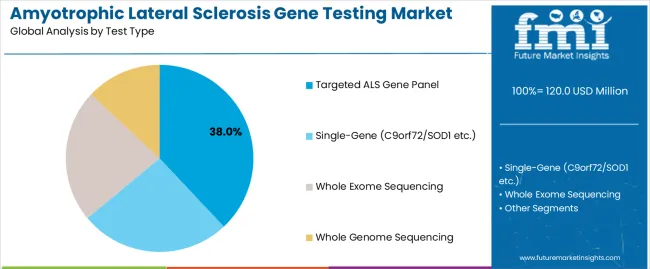
The targeted ALS gene panel segment is projected to account for 38% of the amyotrophic lateral sclerosis gene testing market in 2025, reaffirming its position as the leading test type category. Healthcare facilities and genetic testing integrators increasingly utilize gene panel implementations for their superior diagnostic coverage characteristics when operating across diverse patient platforms, excellent mutation detection properties, and widespread acceptance in applications ranging from basic ALS diagnosis to premium research operations. Gene panel technology's established sequencing methods and proven diagnostic capabilities directly address the facility requirements for dependable testing solutions in complex clinical environments.
This test type segment forms the foundation of modern neuromuscular center adoption patterns, as it represents the implementation with the greatest market penetration and established physician acceptance across multiple patient categories and clinical segments. Facility investments in gene panel standardization and testing consistency continue to strengthen adoption among neuromuscular providers and genetic clinics. With healthcare providers prioritizing diagnostic accuracy and clinical utility, gene panel implementations align with both functionality preferences and cost expectations, making them the central component of comprehensive genetic testing strategies.
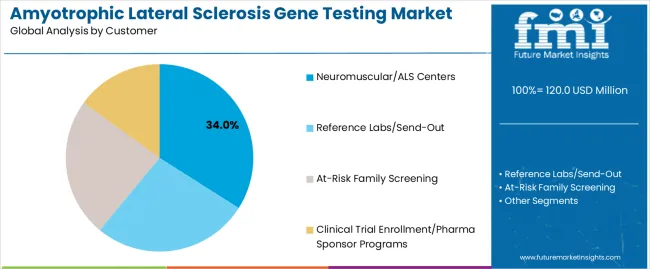
Neuromuscular/ALS center applications are projected to represent 34% of ALS gene testing demand in 2025, underscoring their critical role as the primary customer type for genetic screening across specialized clinical operations. Healthcare facilities prefer ALS gene testing for neuromuscular center use for their exceptional diagnostic characteristics, scalable testing options, and ability to enhance patient management while ensuring consistent clinical outcomes throughout diverse healthcare platforms and patient operations. Positioned as essential diagnostic components for modern neuromuscular systems, gene testing solutions offer both technological advantages and clinical efficiency benefits.
The segment is supported by continuous innovation in genetic testing technologies and the growing availability of specialized implementations that enable diverse neuromuscular requirements with enhanced diagnostic uniformity and extended clinical utility capabilities. Additionally, healthcare facilities are investing in advanced technologies to support large-scale testing integration and research development. As precision medicine trends become more prevalent and genetic awareness increases, neuromuscular center applications will continue to represent a major implementation market while supporting advanced clinical utilization and technology integration strategies.
The blood segment is expected to capture 52% of the amyotrophic lateral sclerosis gene testing market in 2025, driven by increasing demand for reliable sample collection systems that enhance diagnostic accuracy while maintaining clinical convenience. Healthcare providers are increasingly utilizing blood samples for ALS gene testing due to their superior DNA quality, established collection protocols, and clinical familiarity. The segment benefits from growing clinical adoption requirements and continuous innovation in blood-based testing formulations tailored for genetic applications.
The amyotrophic lateral sclerosis gene testing market is advancing rapidly due to increasing awareness of ALS genetic factors and growing adoption of genetic testing technologies that provide superior diagnostic characteristics and clinical benefits while enabling enhanced patient outcomes across diverse neuromuscular and research applications. The market faces challenges, including complex regulatory requirements, evolving clinical guidelines, and the need for specialized genetic expertise and counseling programs. Innovation in sequencing approaches and advanced genomic systems continues to influence product development and market expansion patterns.
Expansion of Precision Medicine Technologies and Clinical Integration
The growing adoption of advanced precision medicine solutions, sophisticated genetic capabilities, and diagnostic outcome awareness is enabling genetic testing developers to produce advanced ALS solutions with superior diagnostic positioning, enhanced genetic profiles, and seamless integration functionalities. Advanced precision medicine systems provide improved patient outcomes while allowing more efficient clinical workflows and reliable performance across various healthcare applications and research conditions. Developers are increasingly recognizing the competitive advantages of clinical integration capabilities for market differentiation and testing positioning.
Integration of Advanced Sequencing Methods and Genetic Engineering
Modern genetic testing manufacturers are incorporating advanced sequencing technology, genetic integration, and sophisticated diagnostic solutions to enhance product appeal, enable intelligent screening features, and deliver value-added solutions to healthcare customers. These technologies improve testing performance while enabling new market opportunities, including multi-gene testing systems, optimized sequencing treatments, and enhanced diagnostic characteristics. Advanced genetic integration also allows developers to support comprehensive healthcare technologies and market expansion beyond traditional diagnostic approaches.
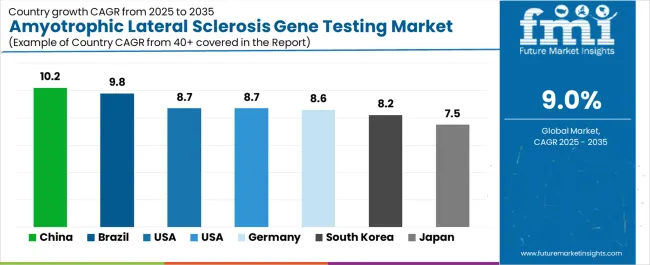
| Countries | CAGR (2025 to 2035) |
|---|---|
| China | 10.2% |
| Brazil | 9.8% |
| USA | 8.7% |
| UK | 8.7% |
| Germany | 8.6% |
| South Korea | 8.2% |
| Japan | 7.5% |
The amyotrophic lateral sclerosis gene testing market is experiencing robust growth globally, with China leading at a 10.2% CAGR through 2035, driven by expanding genetic testing infrastructure, growing neurological research programs, and significant investment in precision medicine development. Brazil follows at 9.8%, supported by increasing healthcare modernization, growing genetic testing integration patterns, and expanding medical genetics infrastructure.
The USA shows growth at 8.7%, emphasizing genetic testing excellence and diagnostic innovation. The UK exhibits 8.7% growth, prioritizing clinical advancement and premium genetic testing development. Germany demonstrates 8.6% growth, focusing on expanding genetic testing capabilities and precision medicine modernization. South Korea records 8.2%, emphasizing genetic innovation excellence and quality-focused clinical patterns. Japan shows 7.5% growth, supported by genetic testing excellence initiatives and quality-focused diagnostic patterns.
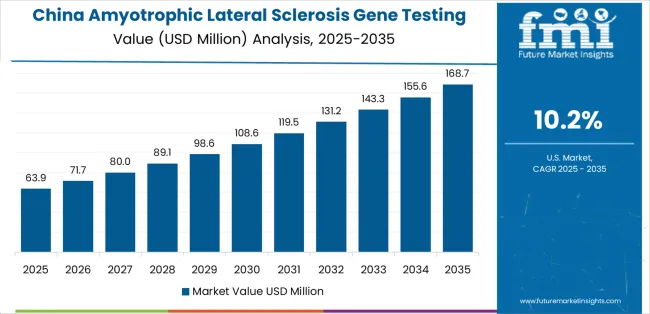
Revenue from amyotrophic lateral sclerosis gene testing in China is projected to exhibit robust growth with a CAGR of 10.2% through 2035, driven by expanding genetic testing infrastructure capacity and rapidly growing precision medicine supported by government initiatives promoting advanced genetic technology development. The country's improving healthcare access and increasing investment in genetic testing infrastructure are creating substantial demand for advanced ALS testing implementations. Major healthcare facilities and genetic testing companies are establishing comprehensive diagnostic capabilities to serve both urban clinical demand and expanding rural healthcare markets.
Government support for precision medicine initiatives and genetic testing development is driving demand for advanced ALS testing systems throughout major healthcare regions and genetic centers across the country. Strong genetic testing growth and an expanding network of specialty-focused providers are supporting the rapid adoption of ALS gene testing among facilities seeking advanced diagnostic capabilities and integrated genetic technologies.
Revenue from amyotrophic lateral sclerosis gene testing in Brazil is growing at a CAGR of 9.8%, driven by the country's expanding healthcare sector, growing genetic testing capacity, and increasing adoption of advanced diagnostic technologies. The country's initiatives promoting healthcare modernization and growing genetic development awareness are driving requirements for technology-integrated testing systems. International genetic testing providers and domestic healthcare companies are establishing extensive clinical and integration capabilities to address the growing demand for advanced ALS testing solutions.
Strong healthcare expansion and expanding modern genetic testing operations are driving adoption of integrated diagnostic systems with superior testing capabilities and advanced integration among large healthcare providers and progressive genetic operations. Growing technology diversity and increasing healthcare enhancement adoption are supporting market expansion for advanced ALS testing implementations with seamless integration profiles and modern diagnostic delivery throughout the country's healthcare regions. Brazil's strategic healthcare position and expanding patient base make it an attractive destination for genetic testing development facilities serving both domestic and Latin American markets.
Revenue from amyotrophic lateral sclerosis gene testing in the USA is growing at a CAGR of 8.7%, driven by the country's focus on genetic innovation advancement, emphasis on premium clinical innovation, and strong position in precision medicine development. The USA's established genetic testing excellence capabilities and commitment to technology diversification are supporting investment in specialized ALS testing technologies throughout major clinical regions. Genetic leaders are establishing comprehensive technology integration systems to serve domestic premium healthcare production and enhancement applications.
Innovations in genetic testing platforms and clinical integration capabilities are creating demand for advanced ALS testing implementations with exceptional diagnostic properties among progressive healthcare facilities seeking enhanced technology differentiation and patient appeal. Growing premium clinical adoption and increasing focus on genetic innovation are driving adoption of advanced testing platforms with integrated diagnostic systems and clinical optimization across healthcare enterprises throughout the country.
Revenue from amyotrophic lateral sclerosis gene testing in the UK is growing at a CAGR of 8.7%, driven by the country's focus on clinical advancement, emphasis on premium genetic innovation, and strong position in precision medicine development. The UK's established clinical excellence capabilities and commitment to technology diversification are supporting investment in specialized ALS testing technologies throughout major healthcare regions. Clinical leaders are establishing comprehensive technology integration systems to serve domestic premium genetic production and enhancement applications.
Innovations in diagnostic platforms and clinical integration capabilities are creating demand for advanced ALS testing implementations with exceptional clinical properties among progressive healthcare facilities seeking enhanced technology differentiation and patient appeal. Growing premium genetic adoption and increasing focus on clinical innovation are driving adoption of advanced testing platforms with integrated diagnostic systems and clinical optimization across genetic enterprises throughout the country.
Revenue from amyotrophic lateral sclerosis gene testing in Germany is expanding at a CAGR of 8.6%, supported by the country's genetic testing heritage, strong emphasis on precision medicine innovation, and robust demand for advanced ALS testing systems in neuromuscular and research applications. The nation's mature healthcare sector and technology-focused operations are driving sophisticated testing implementations throughout the genetic industry. Leading facilities and clinical specialists are investing extensively in genetic development and advanced integration technologies to serve both domestic and international markets.
Rising demand for precision medicine technologies and advanced genetic systems is creating requirements for sophisticated ALS testing solutions with exceptional diagnostic capabilities among quality-conscious facilities seeking enhanced clinical experiences and advanced integration methods. Strong genetic tradition and growing investment in testing technologies are supporting adoption of quality diagnostic platforms with advanced development methods and enhanced genetic profiles across clinical operations in major medical regions.
Revenue from amyotrophic lateral sclerosis gene testing in South Korea is growing at a CAGR of 8.2%, driven by the country's expanding healthcare sector, growing technology integration capacity, and increasing adoption of advanced genetic testing technologies. The country's initiatives promoting healthcare modernization and growing genetic development awareness are driving requirements for technology-integrated testing systems. International genetic providers and domestic healthcare companies are establishing extensive clinical and integration capabilities to address the growing demand for advanced ALS testing solutions.
Rising healthcare requirements and expanding genetic programs are creating opportunities for ALS testing adoption across genetic centers, progressive providers, and modern healthcare facilities in major clinical regions. Growing focus on genetic integration and testing improvement features is driving adoption of testing platforms among providers seeking enhanced diagnostic capabilities and advanced genetic experiences.
Revenue from amyotrophic lateral sclerosis gene testing in Japan is expanding at a CAGR of 7.5%, supported by the country's genetic excellence initiatives, growing quality technology sector, and strategic emphasis on advanced clinical development. Japan's advanced quality control capabilities and integrated healthcare systems are driving demand for high-quality ALS testing platforms in premium applications, genetic technology, and advanced diagnostic applications. Leading facilities are investing in specialized capabilities to serve the stringent requirements of technology-focused healthcare and premium genetic providers.
Quality genetic advancement and technology-focused development are creating requirements for specialized ALS testing solutions with superior quality integration, exceptional diagnostic capabilities, and advanced clinical features among quality-conscious genetic operations and premium healthcare providers. Strong position in genetic technology innovation is supporting adoption of advanced testing systems with validated diagnostic characteristics and quality integration capabilities throughout the country's genetic technology sector.
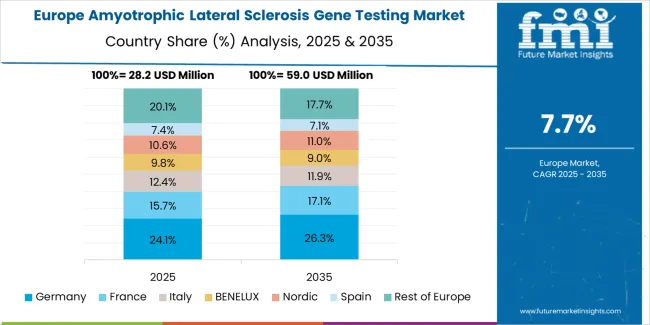
The amyotrophic lateral sclerosis gene testing market in Europe is projected to grow from USD 33.6 million in 2025 to USD 79.5 million by 2035, registering a CAGR of 9% over the forecast period. Germany is expected to maintain its leadership position with a 32% market share in 2025, growing to 33% by 2035, supported by its strong genetic testing culture, sophisticated precision medicine capabilities, and comprehensive healthcare sector serving diverse ALS testing applications across Europe.
France follows with a 20% share in 2025, projected to reach 20.5% by 2035, driven by robust demand for genetic technologies in clinical applications, advanced healthcare development programs, and precision medicine markets, combined with established medical genetics infrastructure and technology integration expertise. The United Kingdom holds a 18% share in 2025, expected to reach 18.5% by 2035, supported by strong genetic technology sector and growing premium healthcare activities. Italy commands a 12% share in 2025, projected to reach 11.5% by 2035, while Spain accounts for 10% in 2025, expected to reach 9.5% by 2035. The Rest of Europe region is anticipated to maintain momentum, with its collective share moving from 8% to 7% by 2035, attributed to increasing genetic modernization and growing technology penetration implementing advanced testing programs.
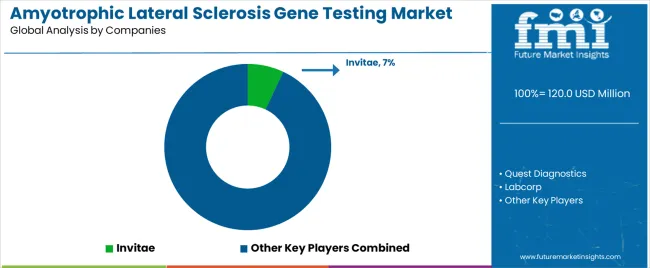
The amyotrophic lateral sclerosis gene testing market is characterized by competition among established genetic testing companies, specialized neurogenetics manufacturers, and integrated diagnostic solution providers. Companies are investing in sequencing technology research, testing optimization, advanced diagnostic system development, and comprehensive genetic portfolios to deliver consistent, high-quality, and patient-specific testing solutions. Innovation in advanced genetic integration, diagnostic enhancement, and clinical compatibility improvement is central to strengthening market position and competitive advantage.
Invitae leads the market with a 7% market share, offering comprehensive genetic testing solutions including quality diagnostic platforms and advanced sequencing systems with a focus on premium and neuromuscular applications. Quest Diagnostics provides specialized laboratory capabilities with an emphasis on advanced ALS testing implementations and innovative diagnostic solutions. Labcorp delivers comprehensive healthcare services with a focus on integrated platforms and large-scale genetic applications. GeneDx specializes in advanced genetic technologies and specialized testing implementations for premium applications. Centogene focuses on neuromuscular-oriented testing integration and innovative genetic solutions.
The competitive landscape is further strengthened by companies like Fulgent Genetics, which brings expertise in advanced genetic sequencing, while Ambry Genetics focuses on specialized genetic testing solutions for rare diseases. Baylor Genetics emphasizes comprehensive genetic testing systems and clinical integration, and Mayo Clinic Laboratories specializes in clinical genetic formulations. These companies continue to invest in research and development, strategic partnerships, and testing capacity expansion to maintain their market positions and capture emerging opportunities in the growing amyotrophic lateral sclerosis gene testing sector.
| Item | Value |
|---|---|
| Quantitative Units | USD 120 million |
| Test Type | Targeted ALS Gene Panel; Single-Gene; Whole Exome Sequencing; Whole Genome Sequencing |
| Customer | Neuromuscular/ALS Centers; Reference Labs/Send-Out; At-Risk Family Screening; Clinical Trial Enrollment/Pharma Sponsor Programs |
| Sample Type | Blood; Saliva/Buccal Swab; Other Biofluids |
| Delivery Model | Hospital Integrated Lab; Commercial Reference Lab; Sponsored/No-Cost Testing Programs; Research/Academic Core Labs |
| Regions Covered | North America; Europe; East Asia; South Asia & Pacific; Latin America; Middle East & Africa |
| Countries Covered | USA; Germany; France; UK; Japan; China; South Korea; Brazil; and 40+ additional countries |
| Key Companies Profiled | Invitae; Quest Diagnostics; Labcorp; GeneDx; Centogene; Fulgent Genetics |
| Additional Attributes | Dollar sales by test type and customer category; regional demand trends; competitive landscape; technological advancements in genetic engineering; advanced sequencing development; diagnostic innovation; clinical integration protocols |
The global amyotrophic lateral sclerosis gene testing market is estimated to be valued at USD 120.0 million in 2025.
The market size for the amyotrophic lateral sclerosis gene testing market is projected to reach USD 284.1 million by 2035.
The amyotrophic lateral sclerosis gene testing market is expected to grow at a 9.0% CAGR between 2025 and 2035.
The key product types in amyotrophic lateral sclerosis gene testing market are targeted als gene panel, single-gene (c9orf72/sod1 etc.), whole exome sequencing and whole genome sequencing.
In terms of customer, neuromuscular/als centers segment to command 34.0% share in the amyotrophic lateral sclerosis gene testing market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Amyotrophic Lateral Sclerosis Market Size and Share Forecast Outlook 2025 to 2035
Lateral Flow Assay Component Market Size and Share Forecast Outlook 2025 to 2035
Lateral Flow Assays Market Analysis - Size, Share & Forecast 2025 to 2035
Unilateral Biportal Endoscopy Market Analysis – Size, Share & Forecast 2024-2034
Patient Lateral Transfer Market - Innovations, Demand & Forecast 2035
The Cryptococcal Antigen Lateral Flow Assay Test Market is segmented by Lateral Flow Readers and Kits and Reagents from 2025 to 2035
Testing, Inspection & Certification Market Growth – Trends & Forecast 2025 to 2035
5G Testing Market Size and Share Forecast Outlook 2025 to 2035
AB Testing Software Market Size and Share Forecast Outlook 2025 to 2035
5G Testing Equipment Market Analysis - Size, Growth, and Forecast 2025 to 2035
Eye Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HSV Testing Market Size and Share Forecast Outlook 2025 to 2035
IoT Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HPV Testing and Pap Test Market Size and Share Forecast Outlook 2025 to 2035
GMO Testing Services Market Insights – Food Safety & Regulatory Compliance 2024 to 2034
GMP Testing Services Market
LTE Testing Equipment Market Growth – Trends & Forecast 2019-2027
Drug Testing Systems Market Size and Share Forecast Outlook 2025 to 2035
Sand Testing Equipments Market Size and Share Forecast Outlook 2025 to 2035
Tire Testing Machine Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA