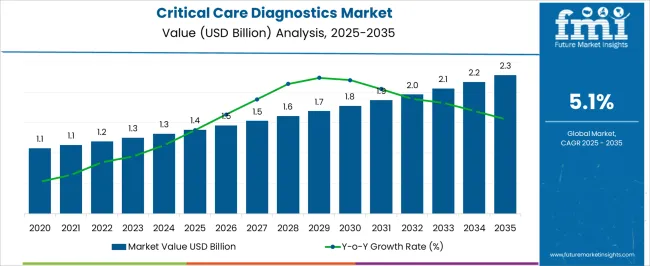
The Critical Care Diagnostics Market is estimated to be valued at USD 1.4 billion in 2025 and is projected to reach USD 2.3 billion by 2035, registering a compound annual growth rate (CAGR) of 5.1% over the forecast period.
| Metric | Value |
|---|---|
| Critical Care Diagnostics Market Estimated Value in (2025 E) | USD 1.4 billion |
| Critical Care Diagnostics Market Forecast Value in (2035 F) | USD 2.3 billion |
| Forecast CAGR (2025 to 2035) | 5.1% |
The Critical Care Diagnostics market is witnessing steady growth, driven by the rising prevalence of chronic diseases, acute medical conditions, and the increasing demand for timely and accurate diagnostic testing. Adoption is being supported by the growing need for rapid, point-of-care diagnostics that enable early detection and intervention, thereby improving patient outcomes. Technological advancements in diagnostic tools, including glucose monitoring kits, immunoassays, and automated analyzers, are enhancing sensitivity, accuracy, and ease of use.
Hospitals, diagnostic laboratories, and clinics are increasingly integrating these solutions into clinical workflows to improve patient management and streamline operations. Regulatory frameworks and clinical guidelines emphasizing early diagnosis and monitoring of critical conditions are further supporting adoption.
Rising awareness among healthcare providers and patients regarding preventive care and continuous monitoring is shaping the market landscape As healthcare infrastructure expands globally and investment in patient-centric technologies increases, the Critical Care Diagnostics market is expected to maintain robust growth, driven by innovations in device technology, digital integration, and scalable diagnostic solutions.
The critical care diagnostics market is segmented by product, prescription mode, end-user, and geographic regions. By product, critical care diagnostics market is divided into Glucose Monitoring Kits, Cardiometabolic Monitoring Kits, Infectious Disease Testing Kits, Coagulation Monitoring Kits, Cancer Markers Testing Kit, Hematology Testing Kits, Pregnancy And Fertility Testing Kits, Cholesterol Test Strips, Drugs-Of-Abuse Testing Kits, Fecal Occult Testing Kits, and Other Critical Care Diagnostics Testing Kits. In terms of prescription mode, critical care diagnostics market is classified into Prescription-Based Testing Kits and OTC Testing Kits Market. Based on end-user, critical care diagnostics market is segmented into Hospitals, Ambulatory Surgical Centres, and Research Laboratories. Regionally, the critical care diagnostics industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
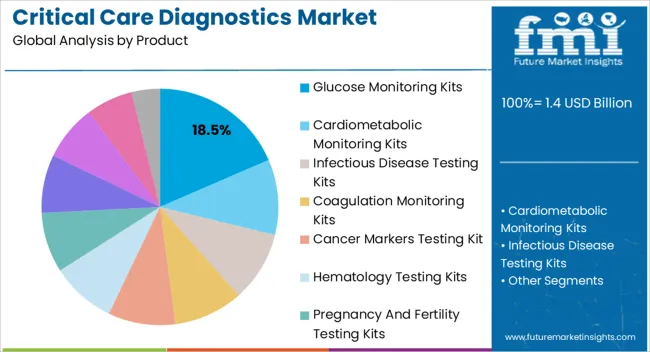
The glucose monitoring kits product segment is projected to hold 18.5% of the market revenue in 2025, positioning it as a leading diagnostic tool. Its dominance is driven by the high prevalence of diabetes and the critical need for continuous blood glucose monitoring in both inpatient and outpatient settings. These kits enable rapid and accurate assessment of glucose levels, supporting timely therapeutic interventions and improving clinical outcomes.
The convenience, portability, and ease of use of modern glucose monitoring kits have accelerated adoption in hospitals, clinics, and home care settings. Integration with digital platforms and data management systems allows healthcare providers to track patient trends and optimize treatment plans efficiently. Advancements in biosensor technology and test strip accuracy further enhance reliability and clinical acceptance.
Continuous awareness campaigns and initiatives by healthcare institutions to promote diabetes management have strengthened market demand As the focus on preventive care and real-time monitoring grows, glucose monitoring kits are expected to retain a significant share in the critical care diagnostics landscape, driven by their clinical relevance and ease of deployment.
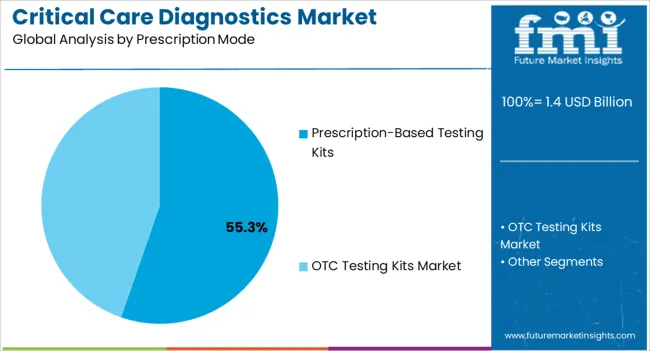
The prescription-based testing kits segment is anticipated to account for 55.3% of the market revenue in 2025, making it the dominant prescription mode. Its growth is supported by stringent regulatory and clinical guidelines that require professional oversight for accurate diagnostics and patient safety. These kits provide standardized testing procedures, ensuring reliability and consistency across critical care applications.
Hospitals and diagnostic laboratories prefer prescription-based kits for conditions requiring controlled testing environments, complex analyses, or specialized interpretation. Integration with electronic health records and clinical decision support systems enhances workflow efficiency and patient management. Rising demand for disease monitoring, acute care assessment, and chronic condition management has reinforced adoption.
The ability to deliver accurate, timely results while maintaining compliance with regulatory standards makes prescription-based kits indispensable in critical care diagnostics As healthcare providers continue to emphasize safety, precision, and evidence-based practices, this segment is expected to maintain its market leadership and remain a primary driver of overall growth.
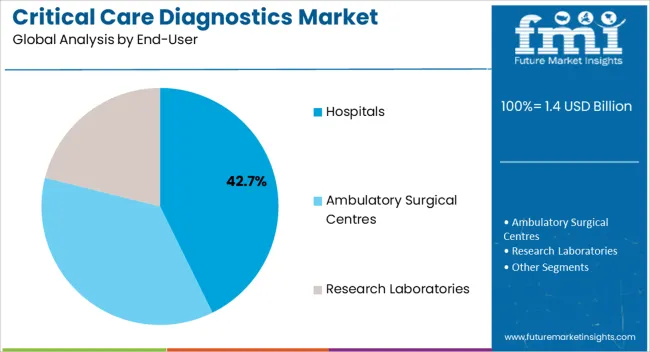
The hospitals end-user segment is projected to hold 42.7% of the market revenue in 2025, establishing it as the leading end-use category. Its dominance is driven by the critical role hospitals play in managing acute and chronic conditions, where rapid and accurate diagnostics are essential for patient care. Hospitals require scalable diagnostic solutions, including glucose monitoring kits and prescription-based testing kits, to handle high patient volumes efficiently.
Integration with hospital information systems and centralized laboratory networks improves workflow efficiency and supports timely clinical decision-making. The growing prevalence of critical illnesses, coupled with increasing hospital admissions and expansion of healthcare infrastructure, further drives demand.
Hospitals also benefit from advancements in automated diagnostics and digital monitoring solutions, which enhance patient management and reduce operational burden As healthcare delivery becomes increasingly patient-centric and technology-driven, hospitals are expected to remain the primary end users in the critical care diagnostics market, supporting sustained adoption and growth of innovative diagnostic solutions.
The global critical care diagnostics market was valued at around US$ 1,133.83 Million at the end of 2025. The market is projected to register a 5.13% CAGR and top a valuation of US$ 1,965.81 Million by 2035.
The market for critical care diagnostics will be driven by factors such as increasing geriatric population, rising prevalence of chronic diseases like diabetes, heart disease, and cancer, and growing awareness of these tests among doctors and patients and their associated advantages.
Point of care diagnostics, also referred to as critical care diagnostics, are authorised tests that are carried out close to the patient and yield fast results. Critical care diagnostics also includes self-testing and over-the-counter tests. Critical care tests are sophisticated tests that deliver results sooner to diagnose the disease for better treatment plans, whereas pathology labs require a set amount of time to provide results depending on the type of test.
While pathology laboratories take hours or even days to produce results, critical care diagnostics offers swift diagnostic testing to the patient and allows for instant results. Blood glucose testing, urine strip testing, blood gas and electrolyte analysis, pregnancy testing, food pathogens screening, drug of abuse, haemoglobin diagnostics, infectious disease testing, and cholesterol screening are all included in the critical care diagnostics.
In addition to being employed at home, clinical laboratories, research facilities, and hospitals all use critical care diagnostics. These diagnostic procedures are economical and offer accurate, prompt diagnosis. These tests are used to diagnose people with life-threatening disorders.
| Attributes | Details |
|---|---|
| Critical Care Diagnostics Market Value in 2025 | US$ 1,133.83 Million |
| Critical Care Diagnostics Market Value in 2035 | US$ 1,965.81 Million |
| Critical Care Diagnostics Market CAGR (2025 to 2035) | 5.13% |
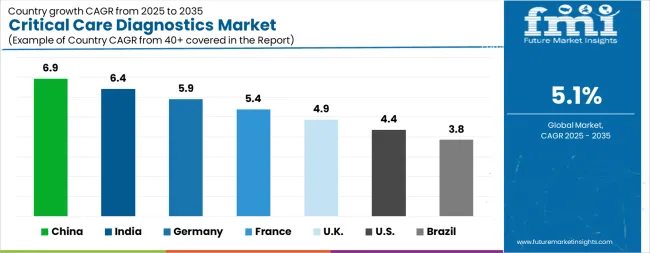
| Country | CAGR |
|---|---|
| China | 6.9% |
| India | 6.4% |
| Germany | 5.9% |
| France | 5.4% |
| UK | 4.9% |
| USA | 4.4% |
| Brazil | 3.8% |
The Critical Care Diagnostics Market is expected to register a CAGR of 5.1% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 6.9%, followed by India at 6.4%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 3.8%, yet still underscores a broadly positive trajectory for the global Critical Care Diagnostics Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 5.9%. The USA Critical Care Diagnostics Market is estimated to be valued at USD 482.4 million in 2025 and is anticipated to reach a valuation of USD 739.2 million by 2035. Sales are projected to rise at a CAGR of 4.4% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 70.5 million and USD 40.7 million respectively in 2025.
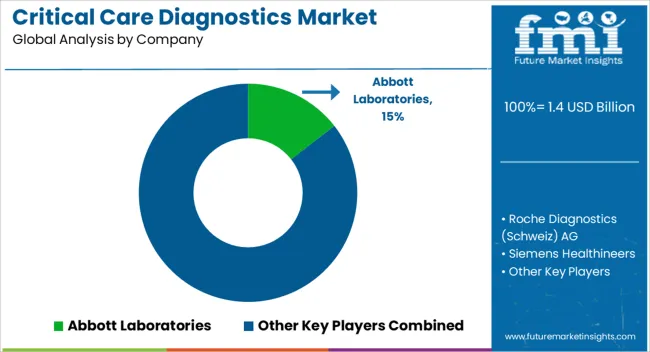
| Item | Value |
|---|---|
| Quantitative Units | USD 1.4 Billion |
| Product | Glucose Monitoring Kits, Cardiometabolic Monitoring Kits, Infectious Disease Testing Kits, Coagulation Monitoring Kits, Cancer Markers Testing Kit, Hematology Testing Kits, Pregnancy And Fertility Testing Kits, Cholesterol Test Strips, Drugs-Of-Abuse Testing Kits, Fecal Occult Testing Kits, and Other Critical Care Diagnostics Testing Kits |
| Prescription Mode | Prescription-Based Testing Kits and OTC Testing Kits Market |
| End-User | Hospitals, Ambulatory Surgical Centres, and Research Laboratories |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
| Key Companies Profiled | Abbott Laboratories, Roche Diagnostics (Schweiz) AG, Siemens Healthineers, Becton, Dickinson & Co., Beckman Coulter, Inc., Bio-Rad Laboratories, Inc., BioMerieux, Inc., Alere, Inc., Bayer Healthcare AG, Sysmex Corporation, Chembio Diagnostic Systems, Inc., EKF Diagnostics Holdings PLC, Nova Biomedical Corporation, Nanomix, Inc., and Biosensors Japan Co., Ltd. |
The global critical care diagnostics market is estimated to be valued at USD 1.4 billion in 2025.
The market size for the critical care diagnostics market is projected to reach USD 2.3 billion by 2035.
The critical care diagnostics market is expected to grow at a 5.1% CAGR between 2025 and 2035.
The key product types in critical care diagnostics market are glucose monitoring kits, cardiometabolic monitoring kits, infectious disease testing kits, coagulation monitoring kits, cancer markers testing kit, hematology testing kits, pregnancy and fertility testing kits, cholesterol test strips, drugs-of-abuse testing kits, fecal occult testing kits and other critical care diagnostics testing kits.
In terms of prescription mode, prescription-based testing kits segment to command 55.3% share in the critical care diagnostics market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Critical Care Patient Monitoring Products Market Forecast and Outlook 2025 to 2035
Critical Care Drugs Market Analysis – Trends, Demand & Forecast 2024-2034
Critical Care Information Systems Market
Point-of-Care Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Point-of-care Molecular Diagnostics Market Insights – Trends & Growth 2025 to 2035
Veterinary Point of Care Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Critical Power and Cooling Market Size and Share Forecast Outlook 2025 to 2035
Critical Infrastructure Monitoring Market
Critical Infrastructure Protection Market
Subcritical CO2 Refrigeration System Market Size and Share Forecast Outlook 2025 to 2035
Suncare Products Market Size and Share Forecast Outlook 2025 to 2035
DNA Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
HIV Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Skincare Supplement Market Size and Share Forecast Outlook 2025 to 2035
Skincare Oil Market Size and Share Forecast Outlook 2025 to 2035
Lip Care Market Analysis - Size and Share Forecast Outlook 2025 to 2035
Skincare Nutritional Serum Market Size and Share Forecast Outlook 2025 to 2035
Haircare Supplement Market - Size, Share, and Forecast Outlook 2025 to 2035
Skincare Products Market Size and Share Forecast Outlook 2025 to 2035
Lip Care Packaging Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA