The global non-invasive jaundice detector market is valued at USD 220.8 million in 2025 and is projected to reach USD 333.2 million by 2035, reflecting a CAGR of 4.2%. Early growth is driven by the increasing prevalence of jaundice in newborns, particularly in developing regions with expanding access to healthcare services. Non-invasive jaundice detectors, which offer quick, reliable, and pain-free screening for neonatal jaundice, are becoming a preferred choice for pediatricians and healthcare providers. The technology's ability to reduce the need for blood tests and its ease of use in both clinical and home settings further drives adoption.
As the market progresses through the forecast period, steady growth continues with increasing awareness of the importance of early jaundice detection in reducing health complications. Advancements in sensor accuracy, portability, and cost-efficiency contribute to broader adoption across healthcare systems and home healthcare environments. Manufacturers also focus on developing devices that integrate with mobile health platforms, enabling real-time data sharing and monitoring. By 2035, the market remains strong, supported by continuous improvements in detection technology, growing healthcare infrastructure, and the ongoing push for early detection of neonatal conditions worldwide.
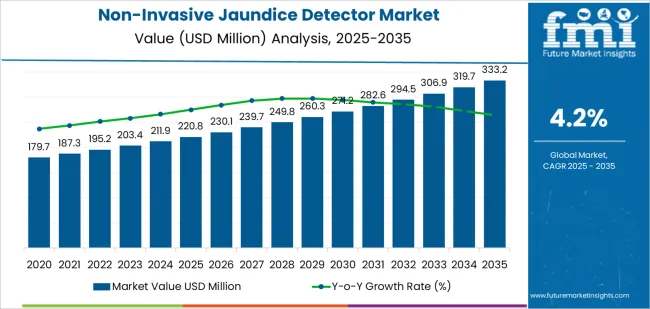
Between 2025 and 2030, the Non-Invasive Jaundice Detector Market grows from USD 220.8 million to USD 271.3 million, creating USD 50.5 million in additional value. The inflection point occurs around 2027 to 2028, when the annual growth rate accelerates from approximately USD 7.3 million to USD 12.7 million, marking a clear shift from steady growth to faster adoption. This inflection reflects increasing demand for non-invasive diagnostic tools, growing awareness of jaundice screening in newborn care, and rising preference for non-invasive procedures over traditional blood tests. Advances in sensor technologies, affordability of devices, and integration with portable health-monitoring systems contribute to this turning point.
From 2030 to 2035, the market expands from USD 271.3 million to USD 333.2 million, adding another USD 61.9 million. The inflection point for this phase occurs between 2032 and 2033, when the market sees a significant increase in adoption, with annual growth moving from USD 12 million to USD 15 million. This later inflection point indicates a stronger market trajectory driven by wider integration of non-invasive jaundice detectors in healthcare systems, especially in developing regions. The device's role in early-stage disease identification, improved affordability, and ease of use in remote settings lead to increased healthcare provider adoption, driving significant market expansion through 2035.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 220.8 million |
| Market Forecast Value (2035) | USD 333.2 million |
| Forecast CAGR (2025 to 2035) | 4.2% |
The non invasive jaundice detector market is growing as hospitals and clinics prioritize tools that measure bilirubin levels without requiring blood draws, thereby reducing discomfort and infection risk in newborns. These devices employ transcutaneous optics and skin tone sensors to detect rising bilirubin in neonates quickly and repeatedly. With about 60% of newborns experiencing some level of jaundice, the need for rapid, safe screening remains high. Manufacturers refine portable form factors, improved calibration algorithms, and integrated monitoring that supports early intervention workflows in nurseries and maternity units. The demand is especially strong in emerging markets, where access to lab based testing is limited and point of care devices help standardize care.
Adoption growth is further supported by regulatory emphasis on early detection of hyperbilirubinemia to prevent complications such as kernicterus. Many hospitals incorporate non invasive jaundice detectors alongside traditional blood tests to expedite decision making and reduce resource utilization. Research into wearable and smartphone based bilirubin monitoring devices is advancing, enabling continuous or home based monitoring for vulnerable infants. Although device cost and reimbursement constraints remain hurdles in some regions, improving accuracy, portability, and ease of integration into neonatal protocols continue to drive adoption and global market growth.
The non-invasive jaundice detector market is segmented by type, application, and region. By type, the market includes non-invasive transcutaneous bilirubin meters, multi-functional jaundice detectors, spectral-based jaundice detectors, jaundice detection modules integrated in monitoring systems, and others. Based on application, it is categorized into neonatal care institutions, pediatric clinics, obstetric hospitals, and others. Regionally, the market is segmented into North America, Europe, East Asia, South Asia, Latin America, and the Middle East & Africa. These divisions reflect differences in clinical workflows, neonatal care capacity, and regional investment in infant health screening technologies.
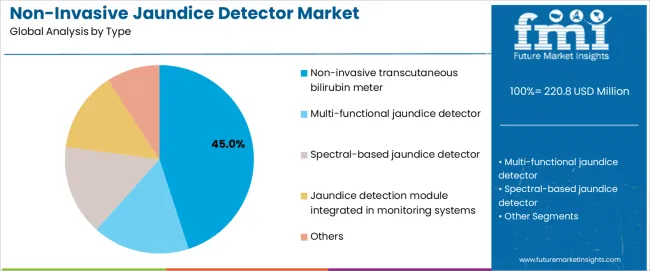
The non-invasive transcutaneous bilirubin meter segment accounts for approximately 45.0% of the global non-invasive jaundice detector market in 2025, making it the leading type category. This position is tied to the segment’s long-established use in neonatal care settings, where quick, painless bilirubin assessments are essential for early detection of jaundice. These meters’ function through optical measurement of bilirubin levels, enabling clinicians to perform rapid screening without blood draws, which is particularly beneficial for premature and newborn infants.
Manufacturers design these devices with calibrated optical sensors that support standardized measurement across different skin tones and neonatal conditions. Adoption is strong in North America, Europe, and East Asia, where neonatal care units routinely screen newborns for jaundice as part of standard postnatal protocols. The segment maintains its leading position because transcutaneous meters are widely recognized for simplicity, speed, and minimal procedural risk, allowing healthcare staff to perform frequent bilirubin checks without disrupting infant care routines.
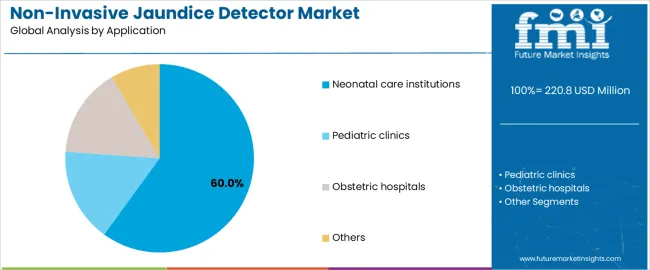
The neonatal care institutions segment represents about 60.0% of the total non-invasive jaundice detector market in 2025, making it the dominant application category. This position reflects the critical role these facilities play in early infant health monitoring, where systematic bilirubin screening helps prevent severe hyperbilirubinemia and related complications. Neonatal units rely on non-invasive devices to conduct repeated measurements during the first days of life, when bilirubin levels frequently change and require close observation.
Hospitals equip neonatal units with multiple detectors to ensure continuous screening capacity, particularly in high-birth-volume regions across East Asia, South Asia, and parts of Europe. These institutions integrate jaundice detection into standardized postnatal workflows, supporting rapid decision-making regarding phototherapy or further diagnostic testing. The segment maintains its lead because neonatal units represent the core environment where bilirubin monitoring is most frequent, creating steady demand for accurate, non-invasive detectors that support infant safety and align with modern neonatal care practices.
The non invasive jaundice detector market is expanding as healthcare providers and caregivers seek safer, quicker and more comfortable alternatives to blood based bilirubin testing for newborns and at risk populations. These devices such as transcutaneous bilirubinometers and optical jaundice meters allow bilirubin assessment through skin contact or imaging, reducing pain and infection risk. Growth is supported by increasing neonatal care standards, rising jaundice incidence globally and technological advances in sensing and analytics. Adoption is restrained by high unit cost, accuracy concerns in diverse skin tones and the need for clinical validation across settings. Device makers are refining algorithms, lowering cost of ownership and improving portability to meet broader use cases.
Demand is driven by the high prevalence of neonatal jaundice affecting a significant percentage of term and pre term infants and clinical imperatives to diagnose early and reduce complications. Hospitals increasingly prefer non invasive tools to monitor bilirubin levels without repeated heel pricks, improving patient comfort and workflow. Care in remote or resource limited settings is also motivating tools that support rapid screening and triage. As healthcare systems expand neonatal units or enforce discharge screening protocols, non invasive jaundice detectors become integral to improving care delivery.
Adoption is constrained by concerns over device accuracy, especially in newborns with darker skin types, or under variable ambient lighting and tissue conditions. Some clinicians remain sceptical of non invasive readings compared to serum bilirubin tests, limiting routine use. The upfront cost of detectors and consumables may be high for smaller clinics or low income regions. Maintenance, calibration and training also add overhead. Regulatory approvals and reimbursement limitations in certain regions further slow adoption, particularly for home care or mobile screening models.
Key trends include miniaturisation and portability, enabling point of care or home based screening; integration of AI and image processing to enhance sensitivity and compensate for skin tone or environmental variability; and development of wearable or continuous monitoring solutions that track bilirubin over time. Device manufacturers are also focusing on cost effective models for emerging markets, cloud based data integration and seamless interoperability with neonatal health information systems. As care models extend into outpatient and home settings, the detector market will continue broadening its reach beyond hospital nurseries.
| Country | CAGR (%) |
|---|---|
| China | 5.7% |
| India | 5.3% |
| Germany | 4.8% |
| Brazil | 4.4% |
| USA | 4.0% |
| UK | 3.6% |
| Japan | 3.2% |
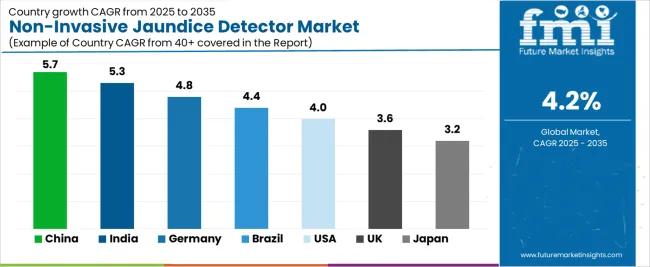
The Non-Invasive Jaundice Detector Market is expanding steadily across healthcare sectors globally, with China leading at a 5.7% CAGR through 2035, driven by significant advancements in neonatal healthcare, increased demand for early jaundice detection, and a growing healthcare infrastructure. India follows at 5.3%, supported by rising awareness, healthcare access improvements, and increasing investment in non-invasive diagnostic technologies.
Germany records 4.8%, benefiting from stringent healthcare standards, advanced medical device manufacturing, and high adoption of neonatal care solutions. Brazil grows at 4.4%, fueled by expanding healthcare access and improved prenatal and neonatal services. The USA, at 4.0%, remains a stable market focusing on pediatric care innovations and home-based diagnostic tools, while the UK (3.6%) and Japan (3.2%) focus on enhancing diagnostic accuracy and patient-friendly solutions for jaundice monitoring.
China is projected to grow at a CAGR of 5.7% through 2035 in the non-invasive jaundice detector market. Increasing healthcare access, neonatal care services, and growing awareness of early disease detection are driving the demand for non-invasive jaundice diagnostic tools. Hospitals and clinics adopt these devices to improve patient comfort and reduce the need for blood tests. Manufacturers focus on enhancing the accuracy and portability of jaundice detectors for widespread use in both rural and urban settings. The market is also supported by government healthcare initiatives promoting early detection and treatment of neonatal conditions.
India is projected to grow at a CAGR of 5.3% through 2035 in the non-invasive jaundice detector market. Growing healthcare infrastructure, awareness programs, and increasing neonatal care facilities contribute to the rising demand for early-stage jaundice detection. Non-invasive devices reduce discomfort and healthcare costs, especially in rural areas where access to laboratory testing is limited. Manufacturers focus on developing affordable, easy-to-use devices to cater to India’s diverse healthcare needs. The market also benefits from mobile health units and government-funded health programs targeting rural populations.
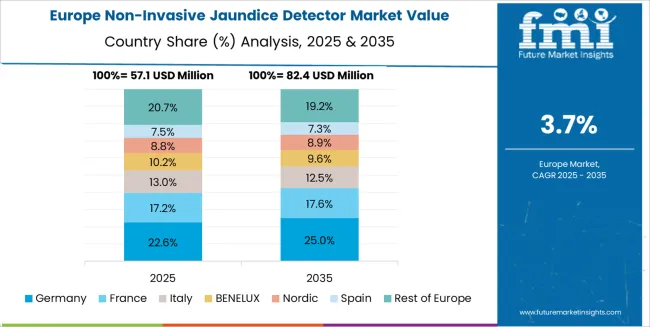
Germany is projected to grow at a CAGR of 4.8% through 2035 in the non-invasive jaundice detector market. As a leader in medical device innovation, Germany’s demand for non-invasive jaundice detection is driven by technological advancements in sensor accuracy and miniaturisation. Hospitals and neonatal care units adopt advanced diagnostic tools to reduce invasive testing and improve patient experience. Manufacturers focus on integrating AI algorithms for enhanced detection and predictive analytics. The market is further driven by Germany’s commitment to healthcare excellence and stringent regulations ensuring high product standards for medical devices.
Brazil is projected to grow at a CAGR of 4.4% through 2035 in the non-invasive jaundice detector market. Increased healthcare access, particularly in rural and underserved regions, drives the adoption of non-invasive diagnostic tools. These devices enable early detection of neonatal jaundice without the need for blood tests, which is crucial for remote or low-resource areas. Manufacturers are focusing on developing cost-effective, portable jaundice detectors that can be used in community health centers. Brazil’s expanding maternal and child healthcare programs also contribute to the market’s growth.
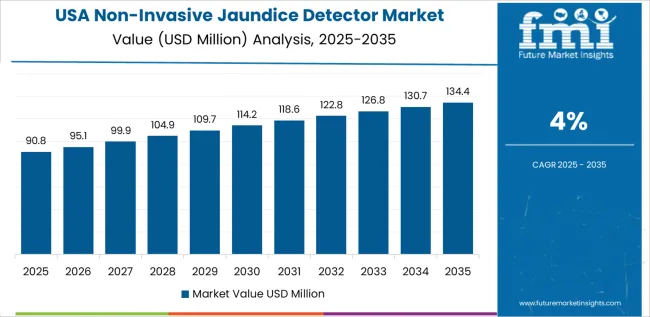
USA is projected to grow at a CAGR of 4% through 2035 in the non-invasive jaundice detector market. The growing adoption of advanced diagnostic technology and the increasing demand for early disease detection are key drivers of market growth. Hospitals and healthcare providers favour non-invasive solutions to reduce the risk of infection and enhance patient comfort. Manufacturers focus on integrating wireless connectivity and cloud data storage for remote monitoring and patient tracking. The USA’s well-established healthcare system continues to invest in innovative diagnostic tools, including non-invasive jaundice detectors.
UK is projected to grow at a CAGR of 3.6% through 2035 in the non-invasive jaundice detector market. National healthcare reforms focusing on reducing healthcare costs and improving patient outcomes are increasing demand for non-invasive diagnostic devices. Hospitals and community health programs adopt jaundice detectors to support neonatal care without the need for blood draws. The UK’s National Health Service (NHS) invests in cost-effective, patient-friendly solutions, encouraging widespread adoption of these technologies. Manufacturers are focusing on enhancing the portability, usability, and accuracy of these devices for use in both clinical and home settings.
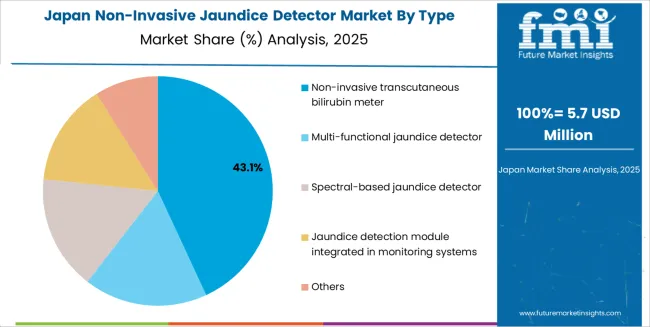
Japan is projected to grow at a CAGR of 3.2% through 2035 in the non-invasive jaundice detector market. Japan’s aging population and high standard of neonatal care increase the need for early detection of conditions such as jaundice. Non-invasive jaundice detectors are used in hospitals and clinics to improve patient comfort and reduce the need for frequent blood tests. Manufacturers focus on developing compact, user-friendly devices for both professional healthcare settings and homecare environments. Japan’s strong healthcare infrastructure supports market expansion, particularly in urban areas with advanced medical facilities.
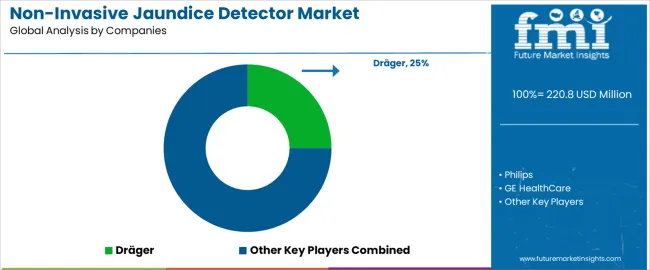
The global non-invasive jaundice detector market is moderately competitive, driven by leading healthcare and medical technology companies providing solutions for neonatal care, routine screenings, and early detection of jaundice in infants. Dräger, Philips, and GE HealthCare hold dominant positions with advanced, clinically validated jaundice detectors that utilize light-based technology to measure bilirubin levels without the need for blood samples. Natus Medical Incorporated and Konica Minolta strengthen the upper tier with reliable and easy-to-use non-invasive solutions that focus on early detection and enhanced clinical outcomes for newborns. Atys Medical, AVI Healthcare, and Micro Lab Instruments contribute to the mid-range competition with portable, user-friendly detectors designed for pediatric and neonatal units in hospitals, clinics, and outpatient settings.
Refine Medical Technology, Beijing M&B Electronic Instruments, Delta Medical International, and Xuzhou Kejian Hi-tech Co., Ltd. expand regional market presence with cost-effective and accurate jaundice detection devices tailored for healthcare providers in emerging markets. Zhengzhou Dison Instrument and Meter Co., Ltd., Shanghai Hisen Limited, and Shenzhen Aeon Technology Co., Ltd. strengthen competition through innovations in sensor accuracy, device portability, and integration with hospital management systems. Market competition is shaped by device reliability, ease of use, and measurement accuracy, while strategic differentiation depends on non-invasive technology, portability, and speed of results. As neonatal healthcare demands rise globally, manufacturers with robust clinical validation, mobile capabilities, and integration with electronic medical records are positioned to strengthen their long-term competitiveness in this growing market.
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD million |
| Type | Non-invasive transcutaneous bilirubin meter, Multi-functional jaundice detector, Spectral-based jaundice detector, Jaundice detection module integrated in monitoring systems, Others |
| Application | Neonatal care institutions, Pediatric clinics, Obstetric hospitals |
| Regions Covered | East Asia, Europe, North America, South Asia, Latin America, Middle East & Africa |
| Countries Covered | China, India, Germany, Brazil, USA, UK, Japan, and 40+ additional countries |
| Key Companies Profiled | Dräger, Philips, GE HealthCare, Natus Medical Incorporated, Konica Minolta, Atys Medical, AVI Healthcare, Micro Lab Instruments, Refine Medical Technology, Beijing M&B Electronic Instruments Co., Ltd., Delta Medical International, Xuzhou Kejian Hi-tech Co., Ltd., Zhengzhou Dison Instrument and Meter Co., Ltd., Shanghai Hisen Limited, Shenzhen Aeon Technology Co., Ltd. |
| Additional Attributes | Market share by type and application, technological advancements (e.g., integration with health platforms, AI and sensor accuracy), regulatory environment across regions, growth in home healthcare, mobile-based jaundice monitoring solutions, emerging market adoption, pediatric and neonatal care trends. |
East Asia
Europe
North America
South Asia
Latin America
Middle East & Africa
Eastern Europe
The global non-invasive jaundice detector market is estimated to be valued at USD 220.8 million in 2025.
The market size for the non-invasive jaundice detector market is projected to reach USD 333.2 million by 2035.
The non-invasive jaundice detector market is expected to grow at a 4.2% CAGR between 2025 and 2035.
The key product types in non-invasive jaundice detector market are non-invasive transcutaneous bilirubin meter, multi-functional jaundice detector, spectral-based jaundice detector, jaundice detection module integrated in monitoring systems and others.
In terms of application, neonatal care institutions segment to command 60.0% share in the non-invasive jaundice detector market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Newborn Jaundice Treatment Market Size and Share Forecast Outlook 2025 to 2035
XEDS Detectors Market Size and Share Forecast Outlook 2025 to 2035
Radar Detectors Market Size and Share Forecast Outlook 2025 to 2035
Flame Detector Market Size and Share Forecast Outlook 2025 to 2035
Smoke Detector Market Insights – Growth & Forecast through 2034
Cable Detector Market
AI-Powered Age Detection – Future of Digital Verification
Quantum Detector Market Size and Share Forecast Outlook 2025 to 2035
Voltage Detector Market Size and Share Forecast Outlook 2025 to 2035
Neutron Detectors Market Analysis - Size, Growth, and Forecast 2025 to 2035
Chemical Detector Market Size and Share Forecast Outlook 2025 to 2035
RF Power Detector Market
Explosive Detectors Market Size and Share Forecast Outlook 2025 to 2035
Total Dust Detector Market Size and Share Forecast Outlook 2025 to 2035
Food Metal Detector Market Size and Share Forecast Outlook 2025 to 2035
Vacuum Leak Detectors Market Size and Share Forecast Outlook 2025 to 2035
Buried Leak Detector Market Size and Share Forecast Outlook 2025 to 2035
Radiometric Detectors Market Size and Share Forecast Outlook 2025 to 2035
HiToxic Gas Detector Market Size and Share Forecast Outlook 2025 to 2035
AI-Powered Deepfake Detection – Protecting Digital Identities

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA