The Chiari malformation treatment market garnered a market value of USD 2.56 billion in 2025 and is expected to accumulate a market value of USD 4.76 billion by registering a CAGR of 6.4% in the forecast period 2025 to 2035.
In 2024, the Chiari malformation treatment industry experienced significant changes and brought improvements in diagnostic technology and surgical procedures. The expansion of applications of advanced imaging technologies, including high-resolution MRIs, made it possible for earlier and more precise diagnosis, leading to more effective treatments. This facilitated the detection of Chiari malformation cases at different stages, ranging from mild to severe, expanding the patient population resorting to medical care.
In the pharma sector, there were moderate advances in drug therapies to manage symptoms of Chiari malformation, but surgical treatment is still the chief intervention. The growing role of patient advocacy groups also contributed significantly to greater awareness and, hence, greater demand for treatments.
The industry will continue to grow steadily during the projection period between 2025 and 2035, driven by ongoing innovation in treatment methods, particularly non-invasive treatments and emerging surgical techniques.
| Metrics | Values |
|---|---|
| Industry Size (2025E) | USD 2.56 Billion |
| Industry Value(2035F) | USD 4.76 Billion |
| CAGR(2025 to 2035) | 6.4% |
The Chiari malformation treatment industry is growth-bound as diagnostic technologies and minimally invasive treatments evolve. As diagnosis rates pick up and awareness grows, demand for surgical and pharmaceutical treatments will increase. Firms investing in cutting-edge treatments and patient-centric care models will gain, but those not embracing developing technologies risk being left behind.
Invest in Minimally Invasive Surgical Innovations
Executives must direct investments in bringing to scale minimally invasive interventions, including endoscopic decompression, which are gaining acceptance because of shorter recovery times and enhanced patient outcomes.
Leverage Advances in Diagnostics and Personalized Medicine
Prioritizing personalized treatment strategies and alliances with diagnostic companies to accelerate early detection will be essential to securing a wider base of patients and maintaining a leadership position.
Enhance Partnerships and M&A in Neurosurgical Research
Partnering with top neurosurgical research centers or buying up startups working on innovative treatments and diagnostics will put companies in a position to leverage upcoming innovations in Chiari malformation treatment and increase their industry share.
| Risk | Probability & Impact |
|---|---|
| Regulatory Changes on Medical Devices | Probability: Medium, Impact: High |
| Slow Adoption of New Surgical Techniques | Probability: Medium, Impact: Medium |
| Supply Chain Disruptions for Advanced Diagnostics | Probability: High, Impact: High |
| Priority | Immediate Action |
|---|---|
| Priority Item 1 | Evaluate feasibility of scaling up minimally invasive surgical techniques. |
| Priority Item 2 | Run feasibility studies on personalized medicine options in Chiari malformation treatment. |
| Priority Item 3 | Engage with diagnostic technology firms to explore partnerships for early detection solutions. |
The industry for Chiari malformation treatments is poised for growth, driven by advancements in both diagnostic and surgical innovations. To maintain a competitive advantage, focus on enhancing minimally invasive surgical options and explore personalized treatment technologies.
Prioritize strategic partnerships with diagnostic firms and invest in early-stage research to solidify a leadership position. Additionally, keeping a close watch on regulatory shifts and global supply chain risks that could impact the scalability of these innovations.
| Country | Regulatory Impact and Mandatory Certifications |
|---|---|
| United States |
|
| European Union |
|
| Japan |
|
| South Korea |
|
| China |
|
| India |
|
| Canada |
|
| Australia |
|
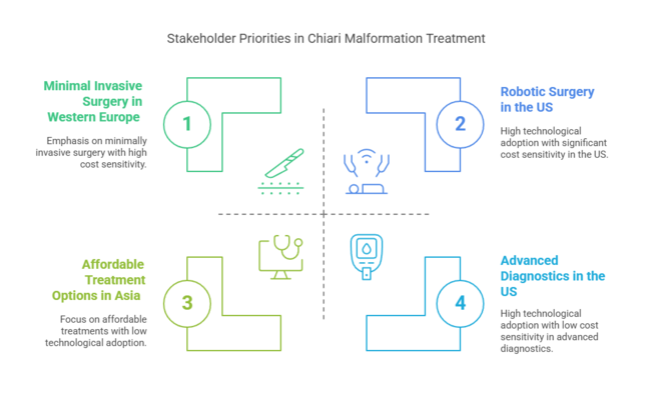
The USA industry for Chiari malformation treatment is expected to develop significantly with a strong healthcare infrastructure, ongoing technological innovation in diagnostic equipment and surgical techniques, and growing use of minimally invasive procedures and robotic interventions. As the industry grows at a higher pace than the worldwide average, the growth is fueled by the increasing use of minimally invasive procedures and robot-assisted treatments.
The need for customized treatment methods and increased awareness in healthcare providers and patients are also primary drivers. Furthermore, the presence of extensive healthcare coverage insurance, including Medicare and Medicaid, drives a higher treatment rate.
FMI opines that the United States chiari malformation treatment sales will grow at nearly 6.8% CAGR through 2025 to 2035.
The UK Chiari malformation treatment industry will grow with the support of improvements in diagnostic technology and surgery. The NHS is the prime driver of access to treatments, though waiting lists and funding limits for healthcare might restrict the adoption of newer therapies. The UK also faces regulatory challenges with the Medicines and Healthcare products Regulatory Agency (MHRA), which can delay the introduction of new devices and treatments.
However, with ongoing research in personalized medicine and minimally invasive treatments, industry growth remains positive, aided by strong public health initiatives and advocacy groups promoting awareness.
FMI opines that the United Kingdom chiari malformation treatment sales will grow at nearly 6.3% CAGR through 2025 to 2035.
France's Chiari malformation treatment industry is driven by the robust healthcare system in the country. The publicly funded healthcare system of France offers widespread coverage for medical procedures, which increases the affordability of Chiari malformation treatments for patients. Nonetheless, the nation struggles with delayed reimbursement for new treatments, which could slow the rapid uptake of new treatments.
However, rising awareness of Chiari malformation, along with investment in further research on minimally invasive surgical techniques and new pharmacological treatments, will drive steady industry growth during the forecast period.
FMI opines that France’s chiari malformation treatment sales will grow at nearly 6.4% CAGR through 2025 to 2035.
Germany's Chiari malformation treatment industry will be supported by the nation's robust healthcare infrastructure, favorable world-class medical facilities, and high emphasis on modern treatment techniques.
Germany is at the forefront in embracing robotic-assisted procedures and cutting-edge diagnostic methodologies, opening up possibilities for faster and precise Chiari treatment. However, regulatory approvals and cost constraints on public healthcare reimbursement may limit rapid adoption, particularly in more expensive treatments.
FMI opines that Germany’s chiari malformation treatment sales will grow at nearly 6.5% CAGR through 2025 to 2035.
Italy’s Chiari malformation treatment industry is forecasted to grow by 2035. The nation has a robust public healthcare system, which grants universal access to healthcare services, enhancing patient access to treatments for Chiari malformation.
The uptake of innovative and advanced treatments can be slower due to bureaucratic processes and limits in the allocation of funds by the National Health Service (NHS). Italy also has regional imbalances in the accessibility of healthcare, which can decelerate overall industry growth.
In spite of these challenges, continued advances in minimally invasive surgical procedures and greater patient exposure will fuel steady industry expansion, although at a slower rate than more developed European economies.
FMI opines that Italy’s chiari malformation treatment sales will grow at nearly 6.2% CAGR through 2025 to 2035.
South Korea's Chiari malformation treatment industry is driven by high government spending on healthcare and the speedy uptake of medical technologies. The nation's emphasis on medical innovation, specifically in the area of robotics and advanced diagnostic methods, caters to the increased need for high-tech Chiari treatments.
The National Health Insurance (NHI) system guarantees wide availability of treatments, although the increasing need for high-end procedures may experience financial limitations in the future. Moreover, the increased consciousness of neurological disorders along with an aging population enhances the incidence of Chiari malformation, which is boosting demand for specialized treatments, thus augmenting industry opportunities.
FMI opines that South Korean chiari malformation treatment sales will grow at nearly 6.6% CAGR through 2025 to 2035.
The industry for Chiari malformation treatment in Japan boasts a well-developed healthcare setup with sophisticated diagnostic equipment and medical devices being readily available. Yet, Japan's highly regulated environment, especially under the Pharmaceuticals and Medical Devices Agency (PMDA), could retard the uptake of new treatments.
In addition, although the aging population enhances the number of diagnoses of Chiari malformation, it is heavily economically constrained to fund the cost of advanced treatments. Despite these challenges, Japan's focus on precision medicine, coupled with growing healthcare investments, is expected to drive moderate but steady growth in the Chiari malformation treatment industry over the next decade.
FMI opines that Japan’s chiari malformation treatment sales will grow at nearly 6.0% CAGR through 2025 to 2035.
China’s Chiari malformation treatment industry is expected to grow at an impressive CAGR of 9.0%, significantly higher than the global average. The size of the patient pool, combined with expanding healthcare expenditures and infrastructure building, makes China a key industry for the treatment of Chiari malformation.
The emphasis from the government to enhance healthcare availability, combined with enhanced awareness for neurological conditions, is likely to boost demand for both surgical and diagnostic procedures. However, issues around reimbursement for costly treatments might constrain accessibility to new technologies. In spite of these challenges, the growth of specialized neurosurgical centers and the growing availability of sophisticated medical devices will drive substantial industry growth.
FMI opines that China’s chiari malformation treatment sales will grow at nearly 7.0% CAGR through 2025 to 2035.
The Australian and New Zealand Chiari malformation treatment industry is driven by the high quality of healthcare systems in the region and access to sophisticated medical treatments. Australia's Medicare system guarantees that the majority of patients are able to receive necessary treatments, though reimbursement for advanced therapies might be limited. New Zealand also has similar issues, with fewer specialized neurosurgical centers.
However, the increasing number of Chiari malformation cases, along with growing public awareness and government efforts to enhance neurological care, will continue to fuel steady industry growth. FMI opines that Australia-NZ chiari malformation treatment sales will grow at nearly 6.1% CAGR through 2025 tio 2035.
The Chiari malformation industry is segmented into four types, wherein all the segments show considerable growth opportunity during the forecast period of 2025 to 2035. Type 1, the most prevalent form, represented the largest segment as it has a higher prevalence and requires more surgical intervention.
The type 2 segment is anticipated to witness significant growth, owing to the rising prevalence of the disease, particularly its early diagnosis that allows a better detection in neonatal and pediatric populations.
Type 3, less common than other types, is also likely to benefit the most from improved awareness and diagnostic technologies, leading to better outcomes. The least common and most serious, type 4 has few treatment options available, but further research into advanced surgical approaches will spur modest growth. Broadly, this segment is expected to grow at a CAGR of 6.5%, similar to what is expected globally.
The Chiari malformation treatment industry is bifurcated into medical and surgical segmentation, with a significant demand expected from 2025 to 2035. Decompression surgery and spinal cord interventions, as part of surgical treatment, have been the predominant treatment option for Chiari malformation and will have a significant contribution to the industry.
The segment will grow at a strong pace as more and more minimally invasive and robotic-assisted procedures emerge. With medical treatments, including pain management, medications, and physical therapy area playing a critical role in supporting patients, these treatments will grow slower than surgical treatments.
In terms of treatment type, the overall treatment segment is projected to witness the fastest growth at a CAGR of 7.0%, owing to increasing access to specialized surgeries along with the adoption of advanced medical technologies.
In general, the diagnostic framework of Chiari malformation relies on MRI, Cine MRI, X-rays, and CT scans, the latter of which is the most utilized device for this purpose, providing extremely detailed imaging of the brain and spinal cord.
The effectiveness of Cine MRI-a technique that is increasingly being recognized for its value to the field of imaging by enabling dynamic analysis of cerebrospinal fluid flow, enabling earlier and more precise diagnosis and follow-through is increasing in line with the growing demand for high-quality early diagnosis from neurologists. X-rays and CT scans are still indispensable for first evaluations, but they are slowly starting to be replaced by much more accurate and thorough MRI scans.
The diagnostic segment for Chiari malformation is growing at a significant CAGR of 7.0% during the forecasted period, owing to advancements in the available technology, combined with an increased awareness regarding early intervention to boost patient outcomes.
Chiari Malformation Treatment Segment by end-user:Hospitals, Surgical centers, Research institutes and Specialty clinics. Hospitals will remain the largest end-user as they can provide full-fledged care from emergency surgeons and long-term management. Of these, surgical centres that focus on neurosurgery will see considerable growth as minimally invasive techniques become increasingly integral to practice.
With that in mind, research institutes will become increasingly involved in the study of Chiari malformation, resulting in breakthroughs in treatments and diagnostics. The overall end-user segment is projected to grow at a CAGR of 6.8%, fuelled by the growing need for higher and advanced health care centers and a more integrated way of treatment.
The treatment industry for Chiari malformation is moderately fragmented, with a number of major players competing to increase their industry share through strategic moves like pricing, innovation, partnerships, and expansion. Players are actively pursuing research and development to launch innovative treatment solutions and are entering into partnerships to increase their distribution channels and industry coverage.
In June 2021, Neurel is, Inc. licensed rights to a portfolio of new ROCK-2 inhibitors for cerebral cavernous malformations to build out its neuroscience pipeline. In May 2023, HAPPE Spine was granted FDA 510(k) clearance for its INTEGRATE-C™ Interbody Fusion System, a system intended to improve healing for spinal surgery.
In 2025, the global Chiari Malformation treatment market is led by Medtronic, commanding approximately 32-35% market share, up from 30% in 2023, driven by their dominance in surgical devices and shunt systems. Integra Life Sciences follows with around 20-22% share, an increase from 18% in 2023, fueled by their expertise in dural grafts and tissue regeneration products used in decompression surgeries.
Stryker Corporation maintains a stable position, accounting for about 15-17% of the market, slightly rising from 14% in 2023, through their strong neurosurgical instruments portfolio. Johnson & Johnson, via DePuySynthes, holds roughly 12-14% share, up from 11% in 2023, bolstered by their cranial stabilization systems.
B. Braun captures around 8-10% share in 2025, improving from 7% in 2023, leveraging their neurosurgical instruments and fluid management solutions. Meanwhile, smaller specialized players such as Olympus Corporation, Zimmer Biomet, and Terumo Corporation collectively represent the remaining 10-15% of the market
It is a brain condition where brain tissue extends into the spinal canal, often causing headaches and other neurological symptoms.
Treatment options include surgical decompression and medical management like pain relief and physical therapy.
MRI, Cine MRI, X-rays, and CT scans are commonly used for diagnosis.
Hospitals, surgical centers, specialty clinics, and research institutes are the main healthcare providers.
North America, Europe, and East Asia are seeing significant growth in treatments for Chiari malformation.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 5: Global Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 9: North America Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 10: North America Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 11: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 12: Latin America Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 13: Latin America Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 14: Latin America Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 15: Latin America Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 16: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 17: Europe Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 18: Europe Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 19: Europe Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 20: Europe Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 21: South Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: South Asia Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 23: South Asia Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 24: South Asia Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 25: South Asia Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 26: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 27: East Asia Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 28: East Asia Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 29: East Asia Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 30: East Asia Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 31: Oceania Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 32: Oceania Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 33: Oceania Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 34: Oceania Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 35: Oceania Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 36: MEA Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 37: MEA Market Value (US$ Million) Forecast by Type, 2018 to 2033
Table 38: MEA Market Value (US$ Million) Forecast by Treatment, 2018 to 2033
Table 39: MEA Market Value (US$ Million) Forecast by Diagnosis , 2018 to 2033
Table 40: MEA Market Value (US$ Million) Forecast by End User, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Type, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 4: Global Market Value (US$ Million) by End User, 2023 to 2033
Figure 5: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 6: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 7: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 8: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 9: Global Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 10: Global Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 11: Global Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 12: Global Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 13: Global Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 14: Global Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 15: Global Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 16: Global Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 17: Global Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 18: Global Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 19: Global Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 20: Global Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 21: Global Market Attractiveness by Type, 2023 to 2033
Figure 22: Global Market Attractiveness by Treatment, 2023 to 2033
Figure 23: Global Market Attractiveness by Diagnosis , 2023 to 2033
Figure 24: Global Market Attractiveness by End User, 2023 to 2033
Figure 25: Global Market Attractiveness by Region, 2023 to 2033
Figure 26: North America Market Value (US$ Million) by Type, 2023 to 2033
Figure 27: North America Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 28: North America Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 29: North America Market Value (US$ Million) by End User, 2023 to 2033
Figure 30: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 31: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 34: North America Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 35: North America Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 36: North America Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 37: North America Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 38: North America Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 39: North America Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 40: North America Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 41: North America Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 42: North America Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 43: North America Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 44: North America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 45: North America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 46: North America Market Attractiveness by Type, 2023 to 2033
Figure 47: North America Market Attractiveness by Treatment, 2023 to 2033
Figure 48: North America Market Attractiveness by Diagnosis , 2023 to 2033
Figure 49: North America Market Attractiveness by End User, 2023 to 2033
Figure 50: North America Market Attractiveness by Country, 2023 to 2033
Figure 51: Latin America Market Value (US$ Million) by Type, 2023 to 2033
Figure 52: Latin America Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 53: Latin America Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 54: Latin America Market Value (US$ Million) by End User, 2023 to 2033
Figure 55: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 56: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 57: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 58: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 59: Latin America Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 60: Latin America Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 61: Latin America Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 62: Latin America Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 63: Latin America Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 64: Latin America Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 65: Latin America Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 66: Latin America Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 67: Latin America Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 68: Latin America Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 69: Latin America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 70: Latin America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 71: Latin America Market Attractiveness by Type, 2023 to 2033
Figure 72: Latin America Market Attractiveness by Treatment, 2023 to 2033
Figure 73: Latin America Market Attractiveness by Diagnosis , 2023 to 2033
Figure 74: Latin America Market Attractiveness by End User, 2023 to 2033
Figure 75: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 76: Europe Market Value (US$ Million) by Type, 2023 to 2033
Figure 77: Europe Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 78: Europe Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 79: Europe Market Value (US$ Million) by End User, 2023 to 2033
Figure 80: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 81: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 82: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 83: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 84: Europe Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 85: Europe Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 86: Europe Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 87: Europe Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 88: Europe Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 89: Europe Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 90: Europe Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 91: Europe Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 92: Europe Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 93: Europe Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 94: Europe Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 95: Europe Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 96: Europe Market Attractiveness by Type, 2023 to 2033
Figure 97: Europe Market Attractiveness by Treatment, 2023 to 2033
Figure 98: Europe Market Attractiveness by Diagnosis , 2023 to 2033
Figure 99: Europe Market Attractiveness by End User, 2023 to 2033
Figure 100: Europe Market Attractiveness by Country, 2023 to 2033
Figure 101: South Asia Market Value (US$ Million) by Type, 2023 to 2033
Figure 102: South Asia Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 103: South Asia Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 104: South Asia Market Value (US$ Million) by End User, 2023 to 2033
Figure 105: South Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 106: South Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 107: South Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 108: South Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 109: South Asia Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 110: South Asia Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 111: South Asia Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 112: South Asia Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 113: South Asia Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 114: South Asia Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 115: South Asia Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 116: South Asia Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 117: South Asia Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 118: South Asia Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 119: South Asia Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 120: South Asia Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 121: South Asia Market Attractiveness by Type, 2023 to 2033
Figure 122: South Asia Market Attractiveness by Treatment, 2023 to 2033
Figure 123: South Asia Market Attractiveness by Diagnosis , 2023 to 2033
Figure 124: South Asia Market Attractiveness by End User, 2023 to 2033
Figure 125: South Asia Market Attractiveness by Country, 2023 to 2033
Figure 126: East Asia Market Value (US$ Million) by Type, 2023 to 2033
Figure 127: East Asia Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 128: East Asia Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 129: East Asia Market Value (US$ Million) by End User, 2023 to 2033
Figure 130: East Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 131: East Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 132: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 133: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 134: East Asia Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 135: East Asia Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 136: East Asia Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 137: East Asia Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 138: East Asia Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 139: East Asia Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 140: East Asia Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 141: East Asia Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 142: East Asia Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 143: East Asia Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 144: East Asia Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 145: East Asia Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 146: East Asia Market Attractiveness by Type, 2023 to 2033
Figure 147: East Asia Market Attractiveness by Treatment, 2023 to 2033
Figure 148: East Asia Market Attractiveness by Diagnosis , 2023 to 2033
Figure 149: East Asia Market Attractiveness by End User, 2023 to 2033
Figure 150: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 151: Oceania Market Value (US$ Million) by Type, 2023 to 2033
Figure 152: Oceania Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 153: Oceania Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 154: Oceania Market Value (US$ Million) by End User, 2023 to 2033
Figure 155: Oceania Market Value (US$ Million) by Country, 2023 to 2033
Figure 156: Oceania Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 157: Oceania Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 158: Oceania Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 159: Oceania Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 160: Oceania Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 161: Oceania Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 162: Oceania Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 163: Oceania Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 164: Oceania Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 165: Oceania Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 166: Oceania Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 167: Oceania Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 168: Oceania Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 169: Oceania Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 170: Oceania Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 171: Oceania Market Attractiveness by Type, 2023 to 2033
Figure 172: Oceania Market Attractiveness by Treatment, 2023 to 2033
Figure 173: Oceania Market Attractiveness by Diagnosis , 2023 to 2033
Figure 174: Oceania Market Attractiveness by End User, 2023 to 2033
Figure 175: Oceania Market Attractiveness by Country, 2023 to 2033
Figure 176: MEA Market Value (US$ Million) by Type, 2023 to 2033
Figure 177: MEA Market Value (US$ Million) by Treatment, 2023 to 2033
Figure 178: MEA Market Value (US$ Million) by Diagnosis , 2023 to 2033
Figure 179: MEA Market Value (US$ Million) by End User, 2023 to 2033
Figure 180: MEA Market Value (US$ Million) by Country, 2023 to 2033
Figure 181: MEA Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 182: MEA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 183: MEA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 184: MEA Market Value (US$ Million) Analysis by Type, 2018 to 2033
Figure 185: MEA Market Value Share (%) and BPS Analysis by Type, 2023 to 2033
Figure 186: MEA Market Y-o-Y Growth (%) Projections by Type, 2023 to 2033
Figure 187: MEA Market Value (US$ Million) Analysis by Treatment, 2018 to 2033
Figure 188: MEA Market Value Share (%) and BPS Analysis by Treatment, 2023 to 2033
Figure 189: MEA Market Y-o-Y Growth (%) Projections by Treatment, 2023 to 2033
Figure 190: MEA Market Value (US$ Million) Analysis by Diagnosis , 2018 to 2033
Figure 191: MEA Market Value Share (%) and BPS Analysis by Diagnosis , 2023 to 2033
Figure 192: MEA Market Y-o-Y Growth (%) Projections by Diagnosis , 2023 to 2033
Figure 193: MEA Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 194: MEA Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 195: MEA Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 196: MEA Market Attractiveness by Type, 2023 to 2033
Figure 197: MEA Market Attractiveness by Treatment, 2023 to 2033
Figure 198: MEA Market Attractiveness by Diagnosis , 2023 to 2033
Figure 199: MEA Market Attractiveness by End User, 2023 to 2033
Figure 200: MEA Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Anorectal Malformation Treatment Market - Trends & Therapeutic Innovations 2025 to 2035
Treatment-Resistant Hypertension Management Market Size and Share Forecast Outlook 2025 to 2035
Treatment-Resistant Depression Treatment Market Size and Share Forecast Outlook 2025 to 2035
Treatment Pumps Market Insights Growth & Demand Forecast 2025 to 2035
Pretreatment Coatings Market Size and Share Forecast Outlook 2025 to 2035
Air Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
CNS Treatment and Therapy Market Insights - Trends & Growth Forecast 2025 to 2035
Seed Treatment Materials Market Size and Share Forecast Outlook 2025 to 2035
Acne Treatment Solutions Market Size and Share Forecast Outlook 2025 to 2035
Scar Treatment Market Overview - Growth & Demand Forecast 2025 to 2035
Soil Treatment Chemicals Market
Water Treatment System Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Chemical Market Size and Share Forecast Outlook 2025 to 2035
Algae Treatment Chemical Market Forecast and Outlook 2025 to 2035
Water Treatment Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Ozone Generator Market Size and Share Forecast Outlook 2025 to 2035
Water Treatment Equipment Market Size and Share Forecast Outlook 2025 to 2035
Burns Treatment Market Overview – Growth, Demand & Forecast 2025 to 2035
CRBSI Treatment Market Insights - Growth, Trends & Forecast 2025 to 2035
Water Treatment Polymers Market Growth & Demand 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA