The CRISPR based gene editing market is valued at USD 4.6 billion in 2025 and projected to reach USD 18.1 billion by 2035, reflecting a CAGR of 14.7%. A rolling CAGR analysis highlights steady compounding growth, as the market more than triples within the forecast horizon. This pace indicates consistent momentum driven by expanding applications in therapeutics, agricultural biotechnology, and functional genomics. The rolling CAGR trend suggests that incremental revenue gains will accelerate after the initial years, supported by rising clinical trial activity and commercialization of targeted therapies. The sustained pace of expansion underscores the market’s long term scalability, with growth dynamics favoring innovation-driven adoption across multiple life science domains.
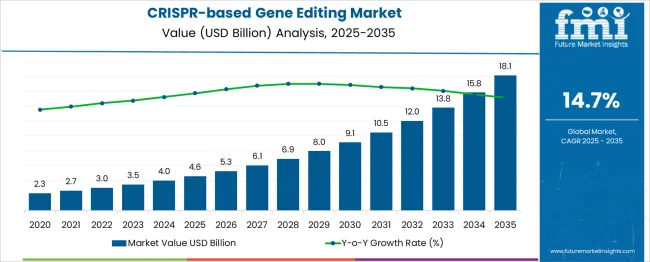
| Item | Value |
|---|---|
| Market Value (2025) | USD 4.6 billion |
| Market Forecast Value (2035) | USD 18.1 billion |
| Market Forecast CAGR (2025–2035) | 14.7% |
The market has been witnessing significant momentum due to its transformative role in biotechnology, pharmaceuticals, and agriculture. Over the past few years, rapid advancements in CRISPR-Cas9 technology have expanded its applications from research laboratories into clinical development and therapeutic pipelines. A clear upward trajectory is visible in the market, with strong investment flows from both public and private sectors. When depicted on a trendline, the market trajectory closely aligns with an exponential growth pattern rather than a linear one, as adoption is accelerating with each breakthrough. Exponential modeling best illustrates how new applications, regulatory approvals, and partnerships are pushing the market beyond steady incremental growth. The sharp curve in this trendline captures the dynamic pace at which CRISPR-based solutions are progressing toward commercialization.
Looking ahead over the next decade, the exponential trendline is expected to remain valid, though polynomial projections may provide additional nuance to capture short-term fluctuations driven by ethical debates, regulatory hurdles, and clinical trial outcomes. While the market may experience some periods of slower movement due to approval delays or safety concerns, the overall direction is upward and transformative. By adopting polynomial trendlines, investors and stakeholders can better visualize potential dips and recoveries within the long-term exponential curve. This perspective reinforces that CRISPR-based gene editing will remain a high-growth domain, propelled by innovations in precision medicine, functional genomics, and agricultural biotechnology.
Market expansion is being supported by the accelerating therapeutic development programs targeting genetic disorders and the corresponding need for precise gene editing tools that can enable effective treatment of previously incurable conditions. Pharmaceutical companies and research institutions across sectors require advanced CRISPR technologies to develop innovative therapies while advancing personalized medicine approaches and improving patient outcomes.
The growing success of clinical trials and increasing regulatory approval of gene editing therapies are driving demand for comprehensive CRISPR platforms that can support diverse research applications and therapeutic development programs. Organizations are investing in advanced gene editing technologies to accelerate drug discovery, improve therapeutic efficacy, and maintain competitive advantages through breakthrough treatment development and commercialization initiatives.
The market is segmented by product & service outlook, application outlook, end use outlook, and region. By product & service outlook, the market is divided into products and services, with products further categorized into CRISPR kits & reagents, CRISPR libraries, and others. Based on application outlook, the market is categorized into biomedical, agricultural applications, plant engineering, farm animals engineering, and others, with biomedical further segmented into therapeutic development and disease diagnostics. In terms of end use outlook, the market is segmented into pharmaceutical & biotechnology companies, academic & research institutes, and contract research organizations. Regionally, the market is divided into China, India, Germany, France, UK, US, and Brazil.
Products are projected to account for 74% of the CRISPR-based Gene Editing market in 2025. This leading share is supported by the widespread demand for CRISPR kits, reagents, libraries, and specialized tools essential for gene editing research and therapeutic development. Product offerings provide the foundational components required for CRISPR applications across research institutions and commercial development programs. The segment benefits from continuous innovation in gene editing tools and comprehensive product portfolios from established biotechnology suppliers.
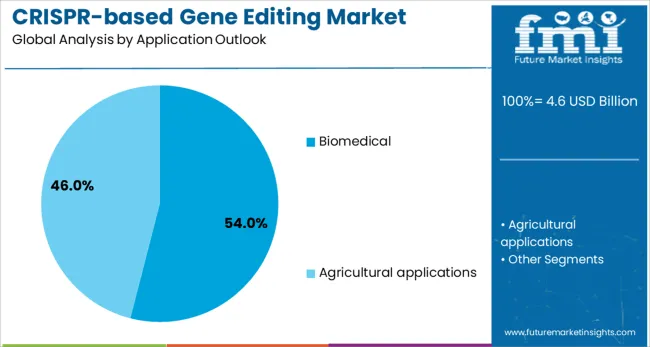
Biomedical applications are expected to represent 54% of CRISPR gene editing demand in 2025. This dominant share reflects the intensive focus on therapeutic development and disease diagnostics using CRISPR technologies across pharmaceutical and biotechnology sectors. Biomedical applications require sophisticated gene editing capabilities for treating genetic disorders, developing personalized therapies, and advancing precision medicine approaches. The segment benefits from substantial research investments and growing clinical validation of CRISPR-based therapeutic approaches.
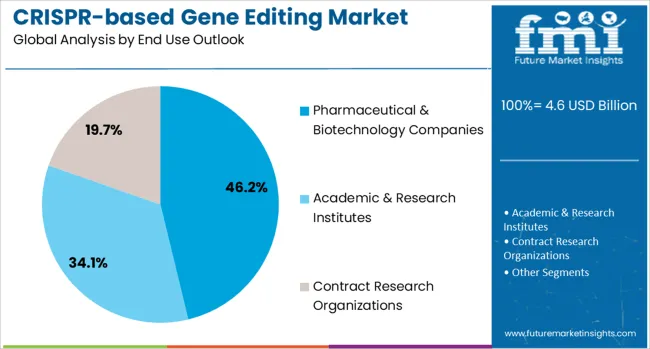
Pharmaceutical & biotechnology companies are projected to contribute 46% of the market in 2025, representing the primary commercial developers and users of CRISPR technologies for therapeutic development programs. These organizations require comprehensive gene editing capabilities for drug discovery, therapeutic development, and clinical trial support across diverse genetic disorder applications. The segment provides significant opportunities for advanced CRISPR platform adoption including research tools, therapeutic development services, and regulatory compliance support.
The CRISPR-based Gene Editing market is advancing rapidly due to accelerating therapeutic development and growing recognition of gene editing potential for treating genetic disorders. The market faces challenges including ethical concerns regarding genetic modification, regulatory complexities for therapeutic applications, and technical limitations in delivery systems. Technological advancement and regulatory framework development continue to influence market growth and development patterns.
The growing success of CRISPR-based therapeutic programs is enabling treatment development for previously incurable genetic disorders while demonstrating clinical efficacy and safety across diverse patient populations. Advanced therapeutic applications are providing breakthrough treatment options for inherited diseases, cancer, and other complex conditions. These developments are particularly valuable for patients with rare genetic disorders and pharmaceutical companies developing precision medicine approaches.
Modern CRISPR developers are incorporating advanced gene editing technologies that improve precision, reduce off-target effects, and enhance delivery capabilities across different cell types and tissue targets. Integration of next-generation editing tools and innovative delivery systems enables more effective therapeutic applications while maintaining safety standards. Advanced systems also support complex gene editing applications including multi-gene modifications and tissue-specific targeting approaches.
| Country | CAGR (2025–2035) |
|---|---|
| China | 19.8% |
| India | 18.4% |
| Germany | 16.9% |
| France | 15.4% |
| United Kingdom | 14.0% |
| United States | 12.5% |
| Brazil | 11.0% |
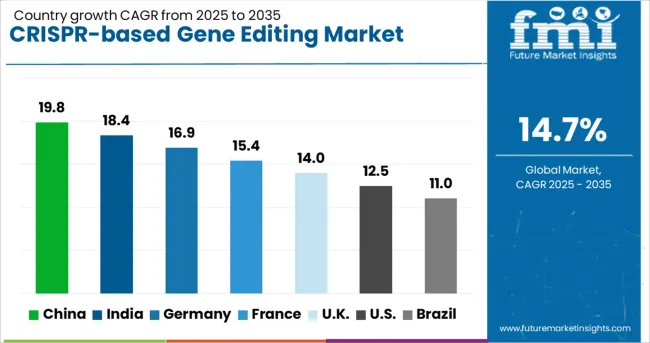
The global CRISPR-based gene editing market is expected to record a CAGR of 14.7% between 2025 and 2035, reflecting its growing role in precision medicine and advanced genetic research. China leads with 19.8% growth, supported by large-scale investments in biotechnology and expanding applications in agriculture and healthcare. India follows at 18.4%, driven by increasing research activity, government funding, and growing adoption in therapeutic development. Germany records 16.9%, reflecting strong focus on clinical trials and advanced genetic engineering initiatives. France is forecast at 15.4%, supported by rising use of CRISPR in pharmaceutical R&D and medical innovations. The United Kingdom grows at 14.0%, with expanding applications in genomics and agricultural sciences, while the United States records 12.5%, influenced by steady commercialization efforts and advanced research programs. Brazil shows 11.0%, driven by expanding biotech capabilities and agricultural applications.
The report covers an in-depth analysis of 40+ countries, top-performing OECD countries are highlighted below.
China is projected to achieve the highest CAGR of 19.8% between 2025 and 2035, driven by large scale investments in biotechnology and national level support for genomic medicine. Research institutes and biotech firms in China have been rapidly expanding CRISPR applications in agriculture, pharmaceuticals, and healthcare. The government has provided strong backing for gene editing research, funding genome centers and clinical trial programs. Chinese firms have prioritized therapeutic pipelines in oncology and rare diseases, while agricultural research centers have used CRISPR to improve crop resilience. Collaboration with global biotech companies has also expanded, allowing Chinese researchers to access cutting edge platforms. Although ethical debates remain significant, China’s comparatively flexible regulatory approach has positioned the country as a major hub for CRISPR innovation over the forecast period.
India is forecasted to grow at a CAGR of 18.4% from 2025 to 2035, with opportunities emerging from both healthcare and agricultural sectors. The adoption of CRISPR has been accelerated by national biotech programs and increased R&D investment in academic institutions. Indian researchers have focused on affordable gene editing applications, particularly in genetic disorders, infectious diseases, and crop yield enhancement. Several collaborations between Indian biotech startups and global CRISPR technology leaders have been observed, expanding access to advanced tools. The regulatory framework is still evolving, but the government has shown willingness to create supportive policies for responsible use. With its large patient population and growing agricultural needs, India has the potential to become a regional leader in translational CRISPR applications.
Germany is projected to grow at a CAGR of 16.9% during 2025–2035, supported by its strong biomedical research base and high healthcare spending. German universities and research institutions have been active in fundamental CRISPR research, with applications extending to personalized medicine and oncology. Pharmaceutical companies have collaborated with academic labs to develop targeted CRISPR therapies, while agricultural research institutions have tested CRISPR for crop resistance and livestock health. Strict regulatory oversight under the European Union has influenced the pace of clinical applications, although academic research remains advanced. Germany’s market outlook remains strong due to its focus on precision medicine and continued investments in genetic research infrastructure.
France is expected to register a CAGR of 15.4% between 2025 and 2035, with growth shaped by government backed research programs and biotech startup activity. French institutions have invested heavily in CRISPR applications for cancer research, rare diseases, and agricultural biotechnology. National laboratories have been involved in collaborative projects with European peers, supported by EU research funding programs. Ethical frameworks have been developed to ensure transparent and responsible use of gene editing, which has influenced both academic and commercial adoption. Startups have been emerging in the French biotech ecosystem, creating competition alongside multinational pharmaceutical companies. France is positioned to play a key role in Europe’s CRISPR research environment, although commercialization may progress at a slower pace than in Asia Pacific regions.
The United Kingdom is forecasted to grow at a CAGR of 14.0% during 2025–2035, with the market supported by the NHS Genomic Medicine Service and leading universities. CRISPR has been applied in translational research programs focused on inherited diseases and oncology. Several biotech companies in the UK have advanced CRISPR based drug discovery platforms, often in collaboration with global pharmaceutical firms. The regulatory environment is well defined, with strict ethical and clinical trial guidelines guiding development. The UK’s role as a European biotechnology hub is strengthened by strong venture funding, which has enabled CRISPR startups to scale operations. While the pace of commercialization is steady, clinical adoption will be cautious given the high ethical scrutiny and rigorous safety requirements in the region.
Brazil is expected to grow at a CAGR of 11.0% between 2025 and 2035, with adoption concentrated in agricultural biotechnology and public health research. CRISPR has been applied by Brazilian research institutions to enhance disease resistance in crops such as soybeans and sugarcane. Healthcare applications have also been emerging, with CRISPR research focusing on neglected tropical diseases and genetic disorders prevalent in the region. Regulatory frameworks are in early development stages, but the government has expressed interest in promoting biotechnology innovation. Collaborations with international research centers have increased Brazil’s participation in global CRISPR initiatives. The outlook remains promising as agricultural and healthcare applications provide long term opportunities for CRISPR based technologies in the country.
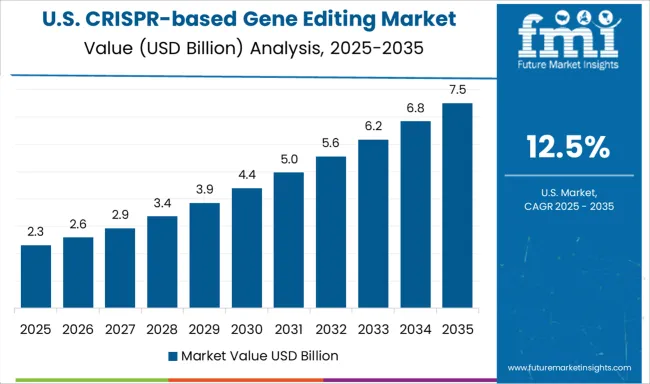
The market in the United States is expected to expand at a CAGR of 12.5% from 2025 to 2035, supported by strong academic research ecosystems and investments from leading biotechnology companies. Clinical trials using CRISPR therapies for rare genetic disorders have been advancing, with regulatory agencies carefully evaluating safety and efficacy. Partnerships between universities, pharmaceutical firms, and CRISPR startups have accelerated translation from lab to clinic. Ethical concerns and strict regulatory oversight have been shaping the adoption pace, especially in germline editing. The competitive landscape has been defined by firms such as CRISPR Therapeutics, Intellia Therapeutics, and Editas Medicine, who are progressing with advanced therapeutic candidates. The USA market is poised to lead in early stage research and commercialization, though regulatory complexity may moderate adoption speed compared to Asia Pacific countries.
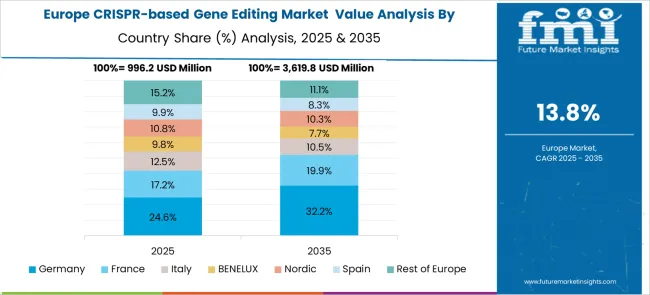
Europe’s CRISPR-based gene editing market is experiencing significant growth driven by robust research and government support. Germany leads the region with a strong focus on precision medicine, genomic research for cancer, and regenerative therapies, growing at a CAGR of around 14.8% through 2030. France shows advanced technology adoption and increasing investment in gene editing research, focusing on therapeutic and agricultural applications. The UK is rapidly expanding, driven by innovation and regulatory support. Other European countries are also increasing focus on CRISPR technology, supported by collaborations between universities, research institutes, and pharmaceutical companies, fueling the market’s
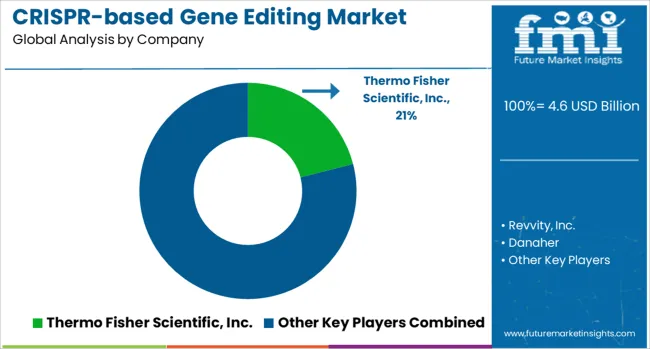
Thermo Fisher Scientific, Revvity, and Danaher have focused on comprehensive CRISPR toolkits, reagents, and delivery systems, enabling applications in functional genomics, drug discovery, and cell therapy. Their strategies focusing reproducibility, platform integration, and partnerships with academic and clinical institutions. Merck KGaA and GenScript have strengthened their positions by offering custom gene editing services, guide RNA libraries, and validated cell lines, ensuring reliable solutions for advanced research.
Specialized providers such as Tocris Bioscience, OriGene Technologies, and Bio-Techne have concentrated on niche segments, including small molecule modulators, CRISPR vectors, and targeted protein validation tools. Bio-Rad Laboratories and New England Biolabs have advanced their roles through high-quality enzymes, delivery technologies, and genome editing kits designed for laboratory efficiency. Product brochures highlight precision editing, off-target minimization, and compatibility with high-throughput screening platforms. Focus is placed on workflow efficiency, ease of use, and regulatory-grade solutions that support translational research.
Competition in the market is about accuracy, scalability, and application breadth. Thermo Fisher and Danaher emphasize integrated platforms for clinical and research use, while GenScript and Merck focus on custom services and tailored editing solutions. Bio-Rad and NEB highlight enzymatic innovation and streamlined workflows. Brochures consistently communicate advantages such as editing fidelity, validated protocols, and application versatility across therapeutics, agriculture, and diagnostics. The market is increasingly defined by technological precision, service reliability, and product adaptability, positioning manufacturers as essential partners in advancing next-generation gene editing research and applications.
| Item | Value |
| Quantitative Units | USD 18.1 billion |
| Product & Service Outlook | Products (CRISPR Kits & Reagents, CRISPR Libraries, Others), Services |
| Application Outlook | Biomedical (Therapeutic Development, Disease Diagnostics), Agricultural applications, Plant Engineering, Farm Animals Engineering, Others |
| End Use Outlook | Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Contract Research Organizations |
| Regions Covered | China, India, Germany, France, United Kingdom, United States, Brazil |
| Country Covered | China, India, Germany, France, United Kingdom, United States, Brazil |
| Key Companies Profiled | Thermo Fisher Scientific Inc., Revvity Inc., Danaher, GenScript, Merck KGaA, Tocris Bioscience, OriGene Technologies Inc., Bio-Rad Laboratories, Bio-Techne, New England Biolabs Inc. |
| Additional Attributes | Dollar sales by product & service outlook, application outlook, and end use outlook, regional demand trends across major biotechnology markets, competitive landscape with established life science companies and specialized gene editing technology providers, integration with therapeutic development programs and clinical trial applications, innovations in gene editing precision and delivery system technologies, and adoption of comprehensive regulatory compliance frameworks for therapeutic development and commercialization programs |
The global crispr-based gene editing market is estimated to be valued at USD 4.6 billion in 2025.
The market size for the crispr-based gene editing market is projected to reach USD 18.1 billion by 2035.
The crispr-based gene editing market is expected to grow at a 14.7% CAGR between 2025 and 2035.
The key product types in crispr-based gene editing market are products, _crispr kits & reagents, _crispr libraries, _others and services.
In terms of application outlook, biomedical segment to command 54.0% share in the crispr-based gene editing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Gene Editing Tool Market Forecast and Outlook 2025 to 2035
Microbial Gene Editing Services Market Size and Share Forecast Outlook 2025 to 2035
Generator Bushing Market Size and Share Forecast Outlook 2025 to 2035
Genetic Analyzers Market Size and Share Forecast Outlook 2025 to 2035
General Intelligent Decision-Making Service Market Size and Share Forecast Outlook 2025 to 2035
General Equipment Rental Services Market Size and Share Forecast Outlook 2025 to 2035
General Purpose DC Contactor Market Size and Share Forecast Outlook 2025 to 2035
Generative AI in Packaging Market Size and Share Forecast Outlook 2025 to 2035
Generative AI In Chemical Market Size and Share Forecast Outlook 2025 to 2035
Generator Sales Market Size and Share Forecast Outlook 2025 to 2035
Generic Injectable Market Report - Growth, Demand & Forecast 2025 to 2035
Generator Sets Market Size and Share Forecast Outlook 2025 to 2035
Generative AI in Logistics Market Size and Share Forecast Outlook 2025 to 2035
Gene Synthesis Market Growth – Trends & Forecast 2025 to 2035
General Surgery Devices Market Insights – Demand and Growth Forecast 2025 to 2035
Gene Prediction Tools Market Insights – Demand and Growth Forecast 2025 to 2035
Gene Therapy in CNS Disorder Market Analysis by Indication, Type, End User, and Region through 2035
Generalized Anxiety Disorder Treatment Market Insights by Drug Class, Therapies, Distribution Channel, and Region 2035
General Purpose Electronic Test and Measurement Instruments Market Analysis and Forecast by Product, End Use, and Region Through 2035
Generalized Myasthenia Gravis Management Market - Growth & Treatment Advances 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA