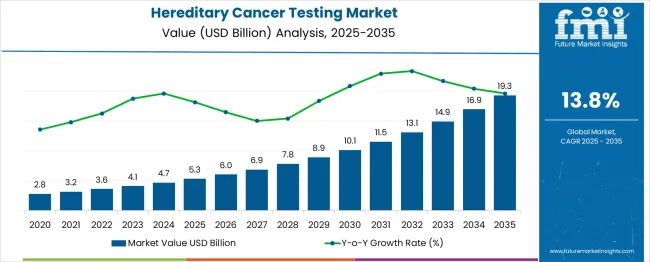
The hereditary cancer testing market is valued at USD 5.3 billion in 2025 and is anticipated to grow to USD 19.3 billion by 2035, registering a CAGR of 13.8%. This initial phase is driven by rising awareness about genetic predispositions to various cancers and advancements in molecular diagnostics. As individuals and healthcare professionals embrace preventive care, the demand for hereditary cancer testing services rises steadily, fueled by early detection capabilities and personalized treatment plans.
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 5.3 billion |
| Forecast Value in (2035F) | USD 19.3 billion |
| Forecast CAGR (2025 to 2035) | 13.8% |
From 2030 to 2035, the market is forecast to grow from USD 10.7 billion to USD 19.3 billion, adding another USD 8.6 billion, which constitutes 61.4% of the ten-year expansion. This period is expected to be characterized by the expansion of pharmacogenomics integration, the development of polygenic risk scores for cancer prediction, and the advancement of liquid biopsy technologies for hereditary cancer monitoring. The growing adoption of direct-to-consumer genetic testing and telemedicine platforms will drive demand for hereditary cancer tests with enhanced accessibility and patient engagement features.
Between 2020 and 2025, the hereditary cancer testing market experienced rapid growth, driven by increased awareness of the genetic factors contributing to cancer and a broader recognition of genetic testing’s clinical value in cancer risk assessment. The ability of genetic testing to provide personalized screening, risk management, and tailored treatment decisions became more widely acknowledged by both healthcare professionals and patients. Advancements in precision medicine, along with the expansion of genetic counseling services, further emphasized the need for hereditary cancer testing, positioning it as a crucial component of modern cancer care and prevention strategies.
Market expansion is being supported by the increasing recognition of genetic factors in cancer development and the corresponding demand for genetic testing services that can identify individuals at high risk for hereditary cancer syndromes. Modern healthcare systems are increasingly focused on preventive medicine approaches that can identify at-risk individuals before cancer development and enable targeted screening and risk reduction strategies. Hereditary cancer testing's proven ability to detect pathogenic genetic variants and guide personalized cancer prevention makes it an essential technology for precision medicine and preventive oncology.
The growing focus on personalized medicine and targeted therapy selection is driving demand for hereditary cancer testing that can inform treatment decisions and identify patients who may benefit from specific therapeutic approaches. Healthcare providers' preference for testing solutions that combine clinical utility with genetic counseling support is creating opportunities for comprehensive genetic testing services. The rising influence of direct-to-consumer genetic testing and patient empowerment trends is also contributing to increased adoption of hereditary cancer testing across diverse patient populations.
The market is segmented by cancer type, technology, test type, end use, and region. By cancer type, the market is divided into breast cancer, lung cancer, colorectal cancer, cervical cancer, ovarian cancer, prostate cancer, stomach/gastric cancer, melanoma, sarcoma, uterine cancer, pancreatic cancer, and others. Based on technology, the market is categorized into molecular testing, cytogenetic, and biochemical. In terms of test type, the market is segmented into predictive testing and diagnostic testing. By end use, the market is classified into hospitals, clinics, and diagnostic centers. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and the Middle East & Africa.
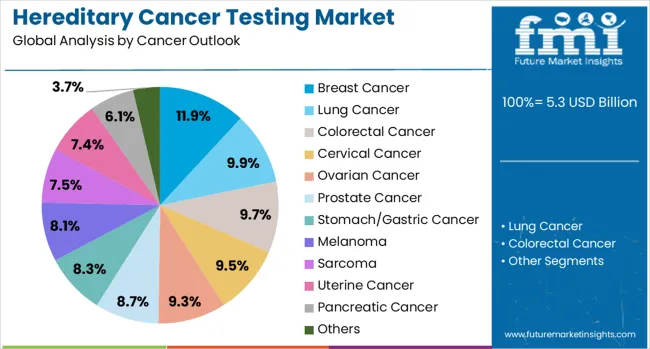
The breast cancer segment is projected to hold a 11.9% share of the hereditary cancer testing market in 2025, reaffirming its dominant position in genetic testing for hereditary cancers. Breast cancer testing, particularly for BRCA1/BRCA2 mutations, has become a well-established clinical tool for identifying individuals at high risk for hereditary breast and ovarian cancer syndromes. The effectiveness of genetic testing in guiding preventive measures, including surveillance and prophylactic surgeries, makes it a cornerstone of hereditary cancer testing programs. Healthcare providers also use breast cancer genetic testing to inform family cascade testing, helping relatives understand their risk. The growing adoption of genetic counseling services further supports the widespread use of breast cancer testing.
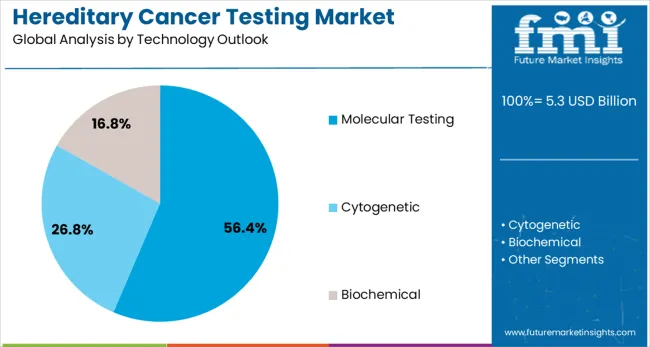
Molecular testing is forecasted to represent 56.4% of hereditary cancer testing demand in 2025.This segment plays a critical role in detecting DNA sequence variations, gene rearrangements, and copy number changes associated with hereditary cancer syndromes. Molecular testing platforms, such as next-generation sequencing (NGS), are highly favored by healthcare providers for their precision and broad genomic coverage. They enable the simultaneous analysis of multiple cancer susceptibility genes, improving the accuracy and comprehensiveness of genetic testing. With ongoing advancements in DNA sequencing technologies, molecular testing continues to evolve, offering greater sensitivity and specificity.
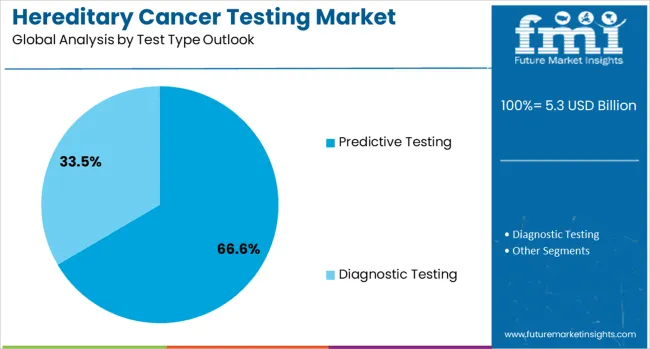
The predictive testing segment is forecasted to account for 66.6% of the hereditary cancer testing market in 2025, reflecting its focus on identifying genetic predispositions in individuals without symptoms. Predictive testing is increasingly used to assess cancer risk in individuals with a family history or clinical risk factors for hereditary cancer syndromes. This aligns with the broader trends in preventive medicine that emphasize early identification of individuals at high risk. By detecting genetic mutations before cancer develops, predictive testing helps healthcare providers develop personalized screening protocols and risk management strategies. The growing recognition of its clinical utility in cancer prevention has made predictive testing a key tool in personalized medicine. With established clinical guidelines supporting its use, predictive testing will remain central to hereditary cancer testing, contributing to early detection, improved outcomes, and the reduction of cancer risk.
The hospital end-use segment is anticipated to contribute 55.2% of the hereditary cancer testing market in 2025, reflecting the primary role of hospital-based healthcare services in delivering comprehensive genetic testing for cancer. Hospitals provide integrated genetic testing services as part of broader cancer care pathways, offering specialized services such as genetic counseling, testing, and clinical management. This integration supports a coordinated approach to hereditary cancer risk assessment and ensures that patients receive tailored care plans based on genetic findings. The hospital setting benefits from established infrastructure, including multidisciplinary teams of genetic counselors, oncologists, and other specialists who can interpret results and guide treatment decisions. As hereditary cancer testing becomes more prevalent, hospitals are central to its delivery, offering comprehensive services and ensuring that patients receive personalized care.
The hereditary cancer testing market is advancing rapidly due to increasing awareness about genetic factors in cancer development and growing adoption of personalized medicine approaches in oncology care. The market faces challenges, including high testing costs, limited genetic counseling resources, and ethical concerns about genetic discrimination and privacy. Innovation in multi-gene panel testing and direct-to-consumer platforms continues to influence product development and market expansion patterns.
The rapid expansion of multi-gene panel testing is significantly enhancing the ability to assess genetic risk for cancer. Unlike traditional single-gene tests, multi-gene panels allow for the simultaneous analysis of multiple genes associated with cancer susceptibility, offering a more comprehensive view of a patient’s genetic risk. This approach not only improves diagnostic accuracy but also helps in the early detection of hereditary cancer syndromes. By analyzing a broader range of genetic mutations, healthcare providers can offer personalized, proactive care plans tailored to individual risk profiles.
The incorporation of artificial intelligence (AI) and machine learning into genetic testing is advancing the field by improving the accuracy of variant interpretation and enhancing risk prediction models. AI algorithms enable more sophisticated analysis of genetic data, facilitating the identification of complex genetic patterns that may not be immediately apparent through traditional methods. These technologies are being used to develop polygenic risk scores, which assess an individual’s likelihood of developing certain types of cancer based on multiple genetic factors.
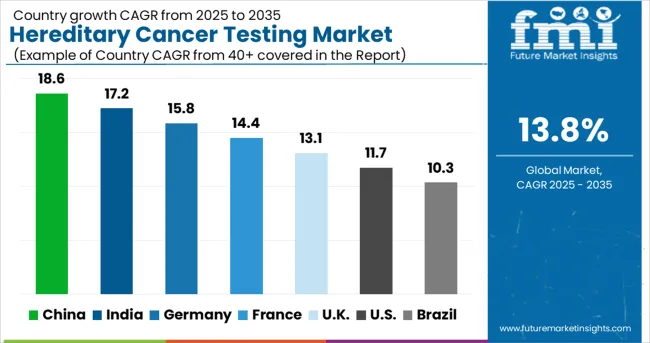
| Country | CAGR (2025 to 2035) |
|---|---|
| China | 18.6% |
| India | 17.2% |
| Germany | 15.8% |
| France | 14.4% |
| UK | 13.1% |
| USA | 11.7% |
| Brazil | 10.3% |
The hereditary cancer testing market is experiencing exceptional growth globally, with a CAGR of 13.8% from 2025 to 2035. China leads the market with an 18.6%, driven by rapidly expanding healthcare infrastructure, increasing cancer awareness, and growing adoption of precision medicine approaches. India follows closely at 17.2%, supported by rising healthcare access, expanding genetic testing capabilities, and increasing awareness about hereditary cancer syndromes. Germany exhibits strong growth at 15.8%, focusing on excellence in genetic medicine and precision oncology. France records a 14.4%, driven by a focus on cancer genetics and comprehensive genetic counseling. The UK shows 13.1% growth, emphasizing NHS genetics programs and cancer prevention initiatives.
The report covers an in-depth analysis of 40+ countries; seven top-performing countries are highlighted below.
Revenue from hereditary cancer testing in China is projected to exhibit exceptional growth with a CAGR of 18.6% through 2035. The rapid development of healthcare infrastructure and rising cancer awareness are key drivers of this growth. Increasing adoption of precision medicine, coupled with government-backed cancer prevention programs, is expanding the demand for genetic testing services. As the middle class grows and healthcare access improves across urban and rural regions, hereditary cancer testing is becoming more accessible. Both domestic and international genetic testing companies are investing heavily to meet the growing demand for advanced genetic solutions. Focus on developing cutting-edge healthcare technologies positions it as a major player in the global hereditary cancer testing market.
India is experiencing significant growth in the hereditary cancer testing market, with a CAGR of 17.2% through 2035. As healthcare access increases, especially in urban areas, more people are seeking personalized cancer prevention services, driving demand for genetic testing. The growing private healthcare sector is crucial in providing specialized services, helping meet the rising demand for hereditary cancer testing. The large and diverse population in India, coupled with an increasing cancer burden, is creating a fertile market for genetic testing solutions. Public awareness of genetic risks is rising, further propelling the adoption of hereditary cancer testing. Domestic and international companies are expanding their reach, capitalizing on this opportunity to deliver affordable genetic testing.
Demand for hereditary cancer testing in Germany is projected to grow at a CAGR of 15.8%. The advanced healthcare system, along with its strong focus on precision oncology, is significantly driving the market. German healthcare providers consistently seek high-quality genetic testing and counseling services, which meet stringent clinical standards. The adoption of multi-gene panels and next-generation sequencing for cancer prevention and screening has been increasing, with a strong emphasis on clinical genetics. As the integration of hereditary cancer testing into routine cancer care continues to grow, demand for these advanced solutions is set to rise. The leadership in genetic medicine further solidifies its position as a key market player.
Revenue from hereditary cancer testing in France is forecasted to expand at a CAGR of 14.4% through 2035. The country’s reputation for excellence in cancer genetics is fueling demand for advanced testing services. French healthcare providers prioritize not just genetic testing, but also comprehensive counseling, which ensures better patient outcomes. Continuous investment in cancer genetics and precision oncology has enabled France to stay at the forefront of genetic testing innovation. The growing collaboration between cancer centers and genetic testing companies is enhancing the development of next-generation testing solutions. France’s strong focus on personalized care and comprehensive patient support continues to drive the market forward.
The hereditary cancer testing market in the UK is anticipated to grow at a CAGR of 13.1% through 2035, driven by the expansion of NHS genetics services and a national focus on cancer prevention. The UK’s healthcare system is investing heavily in genetics programs that aim to provide comprehensive cancer risk assessments and prevention strategies for patients. As more people become aware of their genetic risk factors, demand for hereditary cancer testing has been rising, especially among patients seeking early detection and personalized care. The government’s focus on integrating genetic testing into routine clinical care through the NHS makes these services more accessible to the broader population
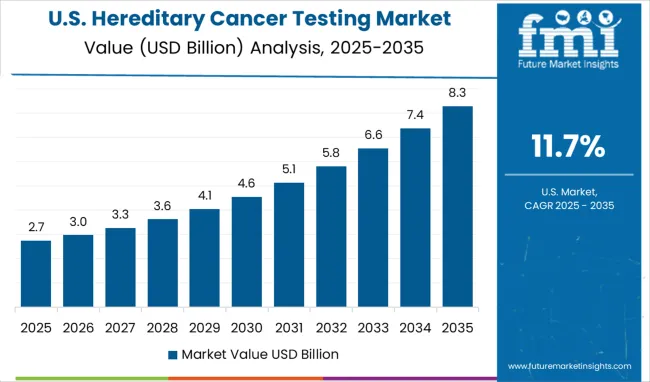
In the United States, the hereditary cancer testing market is expected to expand at a CAGR of 11.7% through 2035, fueled by the country's leadership in genetic testing technology and precision medicine. USA healthcare providers are prioritizing clinical utility and advanced testing methods such as multi-gene panels and liquid biopsies, driving the adoption of hereditary cancer testing. The presence of robust healthcare infrastructure, genetic counseling services, and insurance frameworks is further improving accessibility to testing services for a wide range of patients. The growing emphasis on personalized medicine and early cancer detection is expanding the role of genetic testing in both oncology and preventive care.
Brazil is experiencing a growth rate of CAGR of 10.3% through 2035 in the hereditary cancer testing market, supported by expanding healthcare infrastructure and rising awareness of cancer genetics. As Brazil continues to develop its genetic testing capabilities, demand for hereditary cancer testing services is on the rise, particularly in urban areas where healthcare access is improving. The country’s growing focus on cancer prevention and increasing awareness of hereditary cancer risks among the population is propelling the market forward. Both local and international genetic testing companies are expanding their presence in Brazil, responding to the increasing demand for cancer risk assessments and early detection. Government initiatives to improve healthcare access are positively impacting the growth of genetic testing services.
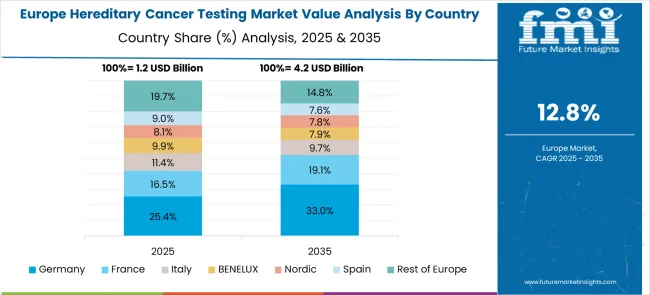
The hereditary cancer testing market in Europe demonstrates advanced development across major economies, with Germany showing a strong presence through its advanced healthcare system and focus on genetic medicine and precision oncology, supported by healthcare providers leveraging clinical genetics expertise to implement comprehensive hereditary cancer testing programs that emphasize patient counseling, clinical utility, and integration with cancer prevention strategies. France represents a significant market driven by its healthcare system excellence and sophisticated understanding of genetic counseling and cancer genetics, with healthcare providers focusing on comprehensive hereditary cancer testing approaches that combine French medical expertise with advanced genetic technologies for enhanced cancer risk assessment and personalized prevention strategies in oncology and genetics services.
The UK exhibits considerable growth through its focus on National Health Service genetics programs and cancer research excellence, with strong adoption of hereditary cancer testing across NHS genetics centers, cancer services, and specialized genetic counseling programs. Germany and France show expanding interest in multi-gene panel testing applications, particularly in comprehensive cancer genetics and personalized prevention strategies. BENELUX countries contribute through their focus on healthcare innovation and genetic medicine advancement. Eastern Europe and Nordic regions display growing potential driven by increasing healthcare investment and expanding genetic testing capabilities.
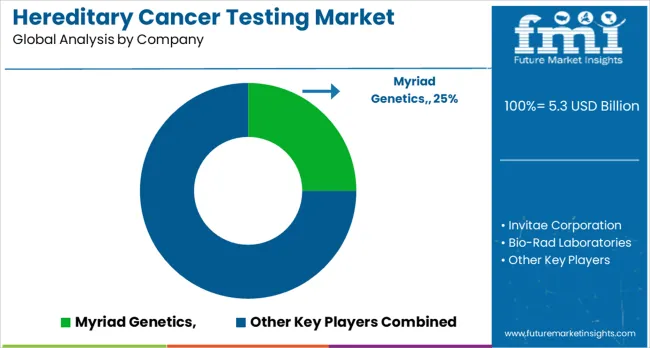
The hereditary cancer testing market is highly competitive, featuring a blend of established genetic testing companies, specialized cancer genetics providers, and emerging direct-to-consumer testing platforms. Companies in this space are heavily investing in test development, clinical validation, and comprehensive patient support services to deliver clinically useful and accurate hereditary cancer testing solutions. Innovations such as multi-gene panel testing, liquid biopsy applications, and AI-assisted interpretation are playing a critical role in improving the accuracy and clinical adoption of these tests.
Myriad Genetics is a leading player in the hereditary cancer testing market, commanding a significant share with its comprehensive testing solutions and a strong emphasis on clinical utility. The company is well-regarded for its integration of genetic counseling services, offering patients valuable support throughout the testing process. Invitae Corporation also stands out by providing accessible genetic testing with a broad menu of tests that cater to a wide range of hereditary cancers.
Invitae integrates genetic counseling, further enhancing its offering by ensuring patients receive the guidance needed to understand their results. Bio-Rad Laboratories is known for its expertise in molecular diagnostic technologies, offering reliable testing solutions that contribute to the accuracy of hereditary cancer detection. CSL Ltd. is another key player, focusing on specialized laboratory services and genetic testing capabilities that support clinical decision-making.
Other significant players, such as QIAGEN NV, Danaher Corporation, Thermo Fisher Scientific, and Abbott Laboratories, play pivotal roles by providing essential molecular testing technologies and laboratory solutions. QIAGEN, for instance, is known for its sample preparation solutions that support genetic testing applications, ensuring high-quality results.
Danaher and Thermo Fisher offer state-of-the-art laboratory instrumentation and diagnostic solutions, while Abbott Laboratories provides innovative molecular diagnostic platforms. Eurofins Scientific, F. Hoffmann-La Roche AG, and Illumina, Inc. further contribute by offering specialized genetic testing technologies and genomic sequencing solutions.
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 5.3 billion |
| Cancer Type | Breast Cancer, Lung Cancer, Colorectal Cancer, Cervical Cancer, Ovarian Cancer, Prostate Cancer, Stomach/Gastric Cancer, Melanoma, Sarcoma, Uterine Cancer, Pancreatic Cancer, Others |
| Technology | Molecular Testing, Cytogenetic, Biochemical |
| Test Type | Predictive Testing, Diagnostic Testing |
| End Use | Hospitals, Clinics, Diagnostic Centers |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Countries Covered | United States, Canada, United Kingdom, Germany, France, China, Japan, South Korea, India, Brazil, Australia and 40+ countries |
| Key Companies Profiled | Myriad Genetics, Invitae Corporation, Bio-Rad Laboratories, CSL Ltd, QIAGEN NV, Danaher Corporation, Thermo Fisher Scientific, Abbott Laboratories, Eurofins Scientific, F. Hoffmann-La Roche AG, and Illumina, Inc. |
| Additional Attributes | Dollar sales by cancer type and test type category, regional demand trends, competitive landscape, buyer preferences for predictive versus diagnostic testing, integration with genetic counseling services, innovations in multi-gene panel testing, AI-assisted interpretation, and direct-to-consumer platform development |
The global hereditary cancer testing market is estimated to be valued at USD 5.3 billion in 2025.
The market size for the hereditary cancer testing market is projected to reach USD 19.3 billion by 2035.
The hereditary cancer testing market is expected to grow at a 13.8% CAGR between 2025 and 2035.
The key product types in hereditary cancer testing market are breast cancer, lung cancer, colorectal cancer, cervical cancer, ovarian cancer, prostate cancer, stomach/gastric cancer, melanoma, sarcoma, uterine cancer, pancreatic cancer and others.
In terms of technology outlook, molecular testing segment to command 56.4% share in the hereditary cancer testing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Hereditary Amyloidosis Treatment Market Size and Share Forecast Outlook 2025 to 2035
Cancer Registry Software Market Size and Share Forecast Outlook 2025 to 2035
Cancer Biological Therapy Market Size and Share Forecast Outlook 2025 to 2035
Cancer Diagnostics Market Analysis - Size, Share and Forecast 2025 to 2035
Cancer Biopsy Market - Growth & Technological Innovations 2025 to 2035
Cancer Vaccines Market Analysis by Technology, Treatment Method, Application and Region from 2025 to 2035
Cancer Gene Therapy Market Overview – Trends & Future Outlook 2024-2034
Cancer Tissue Diagnostic Market Trends – Growth & Industry Forecast 2024-2034
Cancer Supportive Care Products Market Trends – Growth & Forecast 2020-2030
Cancer Antigens Market
Cancer-focused Genetic Testing Service Market Analysis – Growth & Industry Insights 2024-2034
Pet Cancer Therapeutics Market Insights - Growth & Forecast 2024 to 2034
Skin Cancer Detection Devices Market Size and Share Forecast Outlook 2025 to 2035
Lung Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Lung Cancer Surgery Market - Size, Share, and Forecast 2025 to 2035
Lung Cancer Therapeutics Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
Lung Cancer PCR Panel Market Trends, Growth, Demand & Forecast 2025 to 2035
Brain Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Liver Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Blood Cancer Treatment Market Growth – Trends & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA