The global renal denervation catheter market is anticipated to grow from USD 84.9 million in 2025 to approximately USD 690.1 million by 2035, recording an absolute increase of USD 605.2 million over the forecast period. This translates into a total growth of 712.9%, with the market forecast to expand at a CAGR of 23.3% between 2025 and 2035. The market size is expected to grow by nearly 8.1X during the same period, supported by the increasing prevalence of treatment-resistant hypertension and growing adoption of minimally invasive cardiac procedures across global healthcare markets.
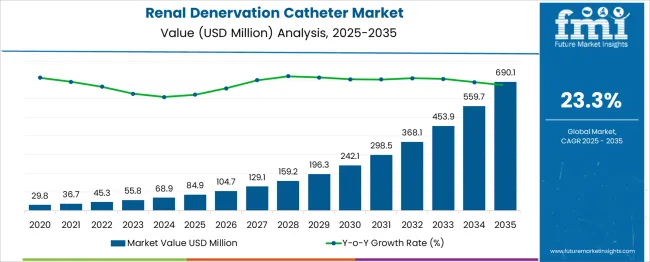
Between 2025 and 2030, the market is projected to expand from USD 84.9 million to USD 242.1 million, resulting in a value increase of USD 157.2 million, which represents 26.0% of the total forecast growth for the decade. This initial growth phase will be influenced by accumulating clinical evidence supporting renal denervation efficacy, rising physician awareness of therapeutic outcomes, and regulatory approvals across leading healthcare regions. Hospitals are expected to strengthen investments in catheter laboratories and clinical training programs, enabling broader integration of renal denervation procedures into treatment pathways.
From 2030 to 2035, the market is forecast to grow from USD 242.1 million to USD 690.1 million, adding another USD 448.0 million, which constitutes 74.0% of the ten-year expansion. This second phase of growth is expected to be characterized by innovation in next-generation catheter platforms, the emergence of ultrasound and radiofrequency-based ablation systems, and clinical validation beyond treatment-resistant hypertension. The increasing adoption of outpatient renal denervation will drive demand for advanced catheter technologies with improved precision, efficiency, and safety profiles.
Between 2020 and 2025, the renal denervation catheter market experienced gradual development, supported by ongoing clinical trials that validated the therapeutic potential of the procedure. Growing recognition of treatment-resistant hypertension as a major global healthcare challenge encouraged further exploration of renal denervation as a viable intervention. Investments in minimally invasive cardiovascular technologies by medical device companies accelerated, with the development of specialized catheter systems for renal artery ablation. Interventional cardiologists and nephrologists began to acknowledge the clinical relevance of the therapy, laying the groundwork for its expansion in the following decade.
| Metric | Value |
|---|---|
| Renal Denervation Catheter Market Value (2025) | USD 84.9 million |
| Renal Denervation Catheter Market Forecast Value (2035) | USD 690.1 million |
| Renal Denervation Catheter Market Forecast CAGR | 33.9% |
Market expansion is being supported by the increasing prevalence of treatment-resistant hypertension and corresponding need for alternative therapeutic approaches that can provide blood pressure reduction without additional medication burden. Modern healthcare systems are recognizing renal denervation as a promising intervention for patients who cannot achieve adequate blood pressure control through conventional pharmaceutical approaches. The growing focuses on personalized medicine and minimally invasive procedures is driving demand for catheter-based interventions that offer targeted therapeutic benefits.
The expanding clinical evidence supporting renal denervation efficacy and improving procedural outcomes are driving adoption among interventional specialists and healthcare institutions. Recent clinical trials demonstrating significant blood pressure reductions and therapeutic benefits have increased physician confidence in renal denervation procedures. Regulatory approvals and clinical guidelines are establishing standardized protocols that support broader implementation of renal denervation in appropriate patient populations.
The growing development of advanced catheter technologies is enabling more precise and efficient renal denervation procedures with improved safety profiles and enhanced therapeutic outcomes. Next-generation catheter systems incorporate advanced imaging guidance, temperature monitoring, and automated ablation protocols that optimize treatment delivery while minimizing procedural risks. These technological improvements are particularly valuable for ensuring consistent treatment results and reducing procedure-related complications that could limit clinical adoption.
Modern renal denervation programs are incorporating comprehensive clinical evidence from large-scale randomized controlled trials that demonstrate blood pressure reductions and cardiovascular benefits. Evidence-based treatment guidelines and patient selection criteria enable physicians to identify appropriate candidates for renal denervation procedures. Clinical registries and outcome tracking systems provide ongoing evidence of real-world effectiveness, supporting continued adoption and reimbursement coverage decisions.
The market is segmented by product type, end use application, and region. By product type, the market is divided into radiofrequency (RF) ablation catheters and ultrasound ablation catheters. Based on end use application, the market is categorized into hospitals and others. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
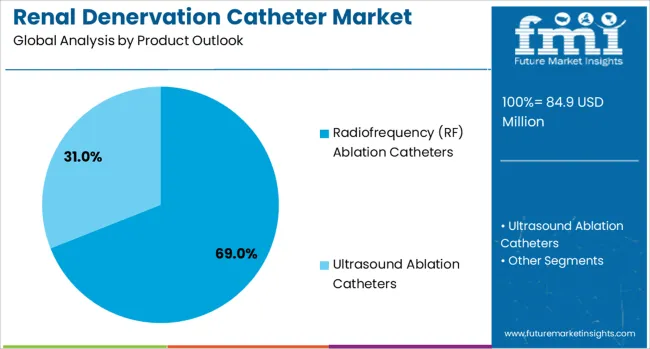
Radiofrequency (RF) ablation catheters are projected to account for 69% of the Renal Denervation Catheter market in 2025. This leading share is supported by the established clinical experience with RF ablation technology and proven efficacy in delivering controlled thermal energy for renal nerve ablation. RF catheter systems offer precise temperature control, real-time monitoring capabilities, and established procedural protocols that facilitate physician adoption and training programs. The segment benefits from extensive clinical data supporting RF ablation effectiveness and well-defined safety profiles that enable regulatory approval and clinical implementation.
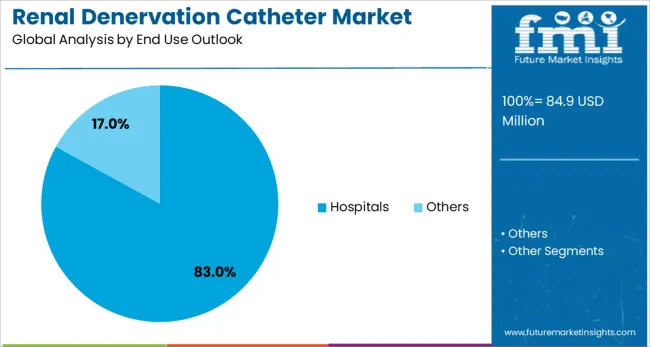
Hospitals are expected to represent 83.0% of renal denervation catheter utilization in 2025. This dominant share reflects the requirement for specialized catheter laboratory facilities, trained interventional specialists, and comprehensive patient monitoring capabilities that are typically available in hospital settings. Hospital-based renal denervation programs offer comprehensive care coordination, emergency response capabilities, and multidisciplinary team support, ensuring optimal patient outcomes. The segment benefits from established reimbursement frameworks and institutional support for advanced cardiovascular intervention programs.
The market faces challenges, including limited long-term outcome data, complex patient selection criteria, and varying reimbursement coverage across different healthcare systems. Technological advancement and clinical education programs continue to influence adoption patterns and market development.
The growing development of ultrasound-based ablation systems is providing alternative approaches to renal denervation that offer potentially enhanced precision and reduced procedural complexity compared to traditional RF systems. Ultrasound ablation technologies enable non-contact energy delivery and real-time tissue monitoring that may improve treatment consistency and safety profiles. These advanced systems are particularly valuable for addressing anatomical variations and complex renal artery configurations that may present challenges for conventional ablation approaches.
Modern renal denervation procedures are incorporating advanced imaging guidance and navigation systems that provide real-time visualization of catheter positioning and treatment progress. Three-dimensional imaging and fluoroscopic guidance systems enable precise catheter placement and optimal energy delivery to target renal nerve locations. These technological improvements enhance procedural accuracy while reducing radiation exposure and contrast media requirements that benefit both patients and medical staff.
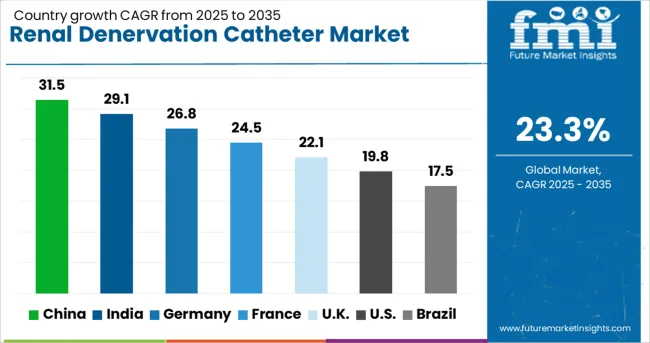
| Countries | CAGR (2025-2035) |
|---|---|
| China | 31.5% |
| India | 29.1% |
| Germany | 26.8% |
| France | 24.5% |
| UK | 22.1% |
| USA | 19.8% |
| Brazil | 17.5% |
The market demonstrates exceptional growth patterns across key countries, with China leading at a 31.5% CAGR through 2035, driven by rapidly expanding healthcare infrastructure, increasing prevalence of hypertension, and growing adoption of advanced cardiovascular intervention technologies. India follows at 29.1%, supported by rising cardiovascular disease burden, expanding specialty care capabilities, and increasing healthcare investment in minimally invasive procedures. Germany maintains strong growth at 26.8%, focusing on clinical excellence and advanced medical technology adoption. France grows at 24.5%, integrating renal denervation with existing cardiovascular care programs. The UK advances at 22.1%, focusing on evidence-based treatment protocols and clinical research programs. The USA shows steady growth at 19.8%, prioritizing regulatory compliance and reimbursement optimization. Brazil records 17.5% growth, driven by expanding healthcare access and cardiovascular intervention capabilities.
Revenue from renal denervation catheters in China is projected to exhibit the highest growth rate with a CAGR of 31.5% through 2035, driven by massive healthcare infrastructure investment, rapidly increasing hypertension prevalence, and growing adoption of advanced cardiovascular intervention technologies across major metropolitan healthcare systems. The country's expanding healthcare capabilities and increasing physician training in interventional procedures are creating significant opportunities for renal denervation adoption. Major hospitals and medical centers are establishing specialized catheter laboratories that support comprehensive cardiovascular intervention programs, including renal denervation procedures.
Government healthcare modernization programs are supporting the development of advanced cardiovascular care capabilities and training programs that enhance domestic expertise in minimally invasive procedures throughout major medical centers and regional hospitals.
Revenue from renal denervation catheters in India is expanding at a CAGR of 29.1%, supported by rising cardiovascular disease burden, expanding specialty care capabilities, and increasing healthcare investment in advanced medical technologies that address complex patient populations. The country's growing medical tourism sector and expanding private healthcare infrastructure are creating opportunities for advanced cardiovascular procedures, including renal denervation. Interventional cardiology programs are gradually establishing capabilities to perform renal denervation procedures for appropriate patient populations.
Medical education and training programs are developing specialized expertise in cardiovascular interventions and hypertension management that support the implementation of advanced therapeutic approaches throughout Indian healthcare systems.
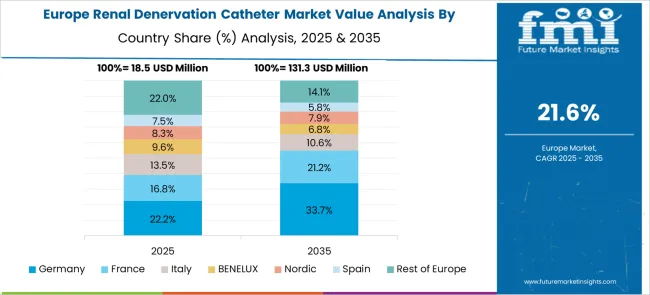
Revenue from renal denervation catheters in Germany is growing at a CAGR of 26.8%, driven by focuses on clinical excellence, comprehensive patient care protocols, and advanced medical technology adoption that supports evidence-based cardiovascular interventions. German cardiovascular centers are implementing renal denervation programs that rigorous patient selection, standardized procedural protocols, and comprehensive outcome tracking. The country's established healthcare system and advanced medical infrastructure provide optimal conditions for implementing sophisticated cardiovascular interventions.
Clinical research programs and evidence-based medicine approaches are ensuring that renal denervation implementation follows established clinical guidelines and maintains high-quality treatment standards throughout German cardiovascular care systems.
Revenue from renal denervation catheters in France is expanding at a CAGR of 24.5%, supported by integration of renal denervation with existing cardiovascular care programs, comprehensive healthcare coverage, and focuses on multidisciplinary treatment approaches that address complex hypertension management requirements. French cardiovascular centers are implementing renal denervation within comprehensive hypertension management programs that include medication optimization, lifestyle interventions, and device-based therapies. Healthcare system coordination enables efficient patient referral and treatment delivery.
Medical specialization and continuing education programs are developing expertise in advanced cardiovascular interventions and ensuring that renal denervation procedures meet established quality standards throughout French healthcare systems.
Revenue from renal denervation catheters in the UK is projected to grow at a CAGR of 22.1%, driven by focuses on evidence-based treatment protocols, comprehensive clinical research programs, and integration of renal denervation within National Health Service cardiovascular care pathways. British cardiovascular centers are implementing renal denervation programs that follow established clinical guidelines and focuses on patient selection criteria based on current evidence. Clinical research initiatives continue to generate evidence supporting renal denervation effectiveness and optimal implementation strategies.
Healthcare system integration and standardized care pathways are enabling efficient renal denervation implementation while maintaining cost-effectiveness and treatment quality throughout British cardiovascular care systems.
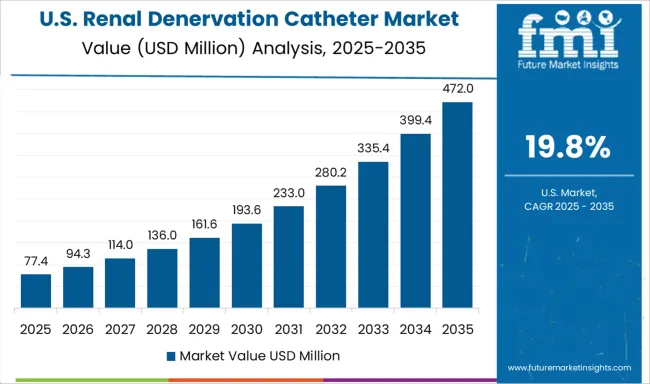
Demand for renal denervation catheters in the USA is expanding at a CAGR of 19.8%, driven by regulatory compliance requirements, reimbursement optimization strategies, and comprehensive clinical evidence supporting renal denervation effectiveness in appropriate patient populations. American cardiovascular centers are implementing renal denervation programs that meet regulatory requirements and demonstrate cost-effectiveness for healthcare payers. Clinical research and registry programs continue to generate real-world evidence supporting renal denervation adoption and reimbursement coverage decisions.
Healthcare technology assessment and value-based care initiatives are ensuring that renal denervation implementation demonstrates clinical effectiveness and economic value throughout American cardiovascular care systems.
Demand for renal denervation catheters in Brazil is growing at a CAGR of 17.5%, supported by expanding healthcare access, increasing cardiovascular disease awareness, and growing investment in advanced medical technologies that address population health needs. Brazilian cardiovascular centers are gradually establishing renal denervation capabilities that serve both private and public healthcare sectors. Medical training programs and technology transfer initiatives are developing domestic expertise in advanced cardiovascular interventions, including renal denervation procedures.
Healthcare infrastructure development and medical education programs are supporting the implementation of advanced cardiovascular care capabilities throughout Brazilian healthcare systems while addressing cost-effectiveness and accessibility requirements.
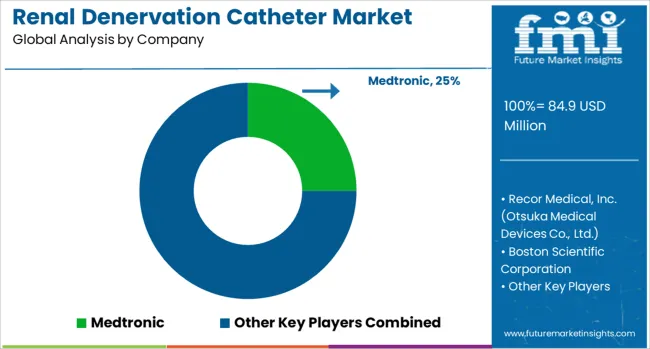
The market is defined by competition among medical device manufacturers, cardiovascular intervention companies, and specialized technology developers. Companies are investing in advanced catheter technologies, clinical evidence generation, regulatory approval processes, and physician training programs to deliver safe, effective, and innovative renal denervation solutions. Strategic partnerships, clinical research collaboration, and market access initiatives are central to strengthening product portfolios and market presence.
Medtronic offers comprehensive renal denervation systems with advanced catheter technologies and established clinical evidence supporting treatment effectiveness. Recor Medical Inc. (Otsuka Medical Devices Co. Ltd.) provides ultrasound-based renal denervation systems with innovative ablation technologies. Boston Scientific Corporation delivers radiofrequency ablation systems with advanced catheter design and procedural support programs. Shanghai Bio-heart Biological Technology Co., Ltd. offers specialized renal denervation catheters designed for Asian markets. MicroPort Scientific Corporation provides comprehensive cardiovascular intervention solutions, including renal denervation systems. EnligHTN offers innovative catheter technologies with a focus on procedural efficiency and safety. ST. JUDE MEDICAL (Abbott Laboratories) delivers established cardiovascular device expertise applied to renal denervation applications. Suzhou Xinmai Medical Technology Co., Ltd. provides specialized catheter systems for renal denervation procedures. Ablative Solutions Inc. offers innovative ablation technologies witha focus on treatment effectiveness and procedural simplicity.
| Items | Value |
|---|---|
| Quantitative Units | USD 84.9 million |
| Product Type | Radiofrequency (RF) Ablation Catheters, Ultrasound Ablation Catheters |
| End Use Application | Hospitals, Others |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Germany, India, China, United Kingdom, France, Brazil |
| Key Companies Profiled | Medtronic, Recor Medical Inc. (Otsuka Medical Devices Co. Ltd.), Boston Scientific Corporation, Shanghai Bio-heart Biological Technology Co. Ltd., MicroPort Scientific Corporation, EnligHTN, ST. JUDE MEDICAL (Abbott Laboratories), Suzhou Xinmai Medical Technology Co. Ltd., Ablative Solutions Inc. |
| Additional Attributes | Dollar sales by product type and end use application, regional demand trends across North America, Europe, and Asia-Pacific, competitive landscape with established medical device companies and emerging technology developers, physician preferences for radiofrequency versus ultrasound ablation technologies, integration with advanced imaging and navigation systems, innovations in catheter design and ablation protocol optimization, and adoption of clinical evidence generation programs with long-term outcome tracking and safety monitoring capabilities. |
The global renal denervation catheter market is estimated to be valued at USD 84.9 million in 2025.
The market size for the renal denervation catheter market is projected to reach USD 690.1 million by 2035.
The renal denervation catheter market is expected to grow at a 23.3% CAGR between 2025 and 2035.
The key product types in renal denervation catheter market are radiofrequency (rf) ablation catheters and ultrasound ablation catheters.
In terms of end use outlook, hospitals segment to command 83.0% share in the renal denervation catheter market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Renal Cyst Treatment Market Size and Share Forecast Outlook 2025 to 2035
Catheter Market Insights by Product, Indication, End-user, and Region 2025 to 2035
Renal Function Test Market Growth - Trends & Forecast 2025 to 2035
Catheter-Directed Thrombolysis Market Growth - Trends & Forecast 2025 to 2035
Catheter Tip Syringe Market Size, Share & Demand Outlook 2025 to 2035
Catheter Associated Urinary Tract Infections (UTI) Treatment Market - Demand & Forecast 2025 to 2035
Renal Insufficiency Treatment Market Analysis by End User, Treatment Type, and Region: Forecast for 2025 to 2035
Renal Biomarker Market Report – Trends & Forecast 2024-2034
Catheter Systems Market
Adrenal Crisis Management Market Analysis and Forecast, By Diagnosis Method, Treatment Method, Distribution Channel, and Region, through 2035
Transcatheter Mitral Valve Market Size and Share Forecast Outlook 2025 to 2035
Transcatheter Heart Valve Replacement Market Analysis - Trends & Forecast 2025 to 2035
Global Microcatheter Market Analysis – Trends, Size & Forecast 2024-2034
Transcatheter Bioprosthesis Market
Foley Catheter Market Analysis - Size, Share, and Forecast 2025 to 2035
Stroke Catheters Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Airway Catheters Market Analysis - Growth & Industry Insights 2025 to 2035
Rectal Catheters Market
Balloon Catheters for Bile Stone Removal Market Size and Share Forecast Outlook 2025 to 2035
Robotic Catheterization Systems Market Growth – Innovations, Trends & Forecast 2025-2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA