A number of infectious diseases and the increasing number of resistant strains of microorganisms lead to the development of microbial detection kits. However these traditional methods of culturing and then identification is time consuming. Automated microbial detection systems is one solution for this problem.
Using these systems the detection process of microorganisms can be done within a few minutes of culturing and the results could be available within a couple of hours. Automated microbial detection systems are automated tools used for the detection of key contaminant microorganisms.
These methods allow for preventive controls and faster testing. The results could be obtained within a few hours using the automated microbial detection systems, when compared to the traditional culturing methods.
A number of large and small players provide automated microbial detection systems. Some of them include Thermo Fisher Scientific, Lonza, Pall Corporation, MediRay, BioMeriux and MIDI Inc.
A number of factors drive the growth of the automated microbial detection systems market. Some of these include the increase in incidence of infectious diseases that are coupled with epidemic and pandemic events that occur every year.
Increasing food safety concerns is another factor that drives the growth of the automated microbial detection system market. Technology advancements and government initiatives to aid the development of the market further.
However, high cost of automated microbial detection systems and complex regulatory framework slightly hinder the growth of the automated microbial detection systems market.
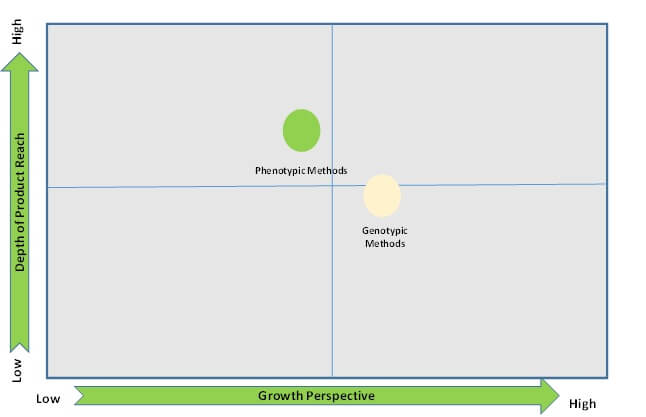
When genotypic methods are applied for the identification of microorganisms it results in specie-specific fingerprints. The genotypic methods of automated microbial detection systems are fast and reliable.
New genotypic methods include the use of microchips coated with an array of oligonucleotides that allows rapid identification, by the determination of patterns or sequences that are specific to an organism.
DNA sequencing and biochemical sequencing coupled with fluorescent analysis of the product is also being developed for automation.
Diagnostic laboratories culture different kinds of samples such as blood and urine for diagnosis of infectious diseases. With the traditional method of culturing and testing the results could take days and the patient may be in distress until then. There is a need to reduce the time required for the detection process. This can be achieved by the use of automated microbial detection systems.
Although North America will continue to hold the largest share in the automated microbial detection systems market, Asia-Pacific is expected to grow with the largest CAGR. The continued focus of the major player towards emerging and Asian countries as well as due to the support of the government in these region leads to development of the automated microbial detection systems market in the region.
Automated Microbial Detection Systems are expensive devices. Thus the Automated Microbial Detection System Market is dominated by large players like Thermo Fisher Scientific, Becton Dickinson, Merck Millipore and Lonza. The other small players include Biomerieux, MIDI Inc and MediRay.
| Small-Scale Manufacturers/Providers | Medium-Scale Manufacturers/Providers | Large-Scale Manufacturers/Providers |
|---|---|---|
|
|
|
FMI utilizes a triangulation methodology that is primarily based on experimental techniques such as patient-level data, number of procedures and capital equipment install base to obtain precise market estimations and insights on various medical devices and medical technology.
Bottom-up approach is always used to obtain insightful data for the specific country/regions. The country specific data is again analysed to derive data at a global level. This methodology ensures high quality and accuracy of information.
Secondary research is used at the initial phase to identify the feasibility of the target products/technology categories and its respective segments, product offerings, usage pattern as per disease indications, product installed base in target healthcare facilities, life span of a device, reimbursement scenario, adoption rate and future impact of new technologies.
Each piece of information is eventually analysed during the entire research project which builds a strong base for the primary research information.
Primary research participants include demand-side users such as key opinion leaders, physicians, surgeons, and supply-side providers of medical devices who provide valuable insights on trends, key treatment patterns, adoption rate, and purchasing pattern, technological development of medical devices, patient education, effectiveness of manufacturers and important strategies, pricing and competitive dynamics.
Quantitative and qualitative assessment of basic factors driving demand, economic factors/cycles and growth rates and strategies utilized by key players in the market is analysed in detail while forecasting, in order to project Year-on-Year growth rates. These Y-o-Y growth projections are checked and aligned as per industry/product lifecycle and further utilized to develop market numbers at a holistic level.
On the other hand, we also analyse various companies annual reports, investor presentations, SEC filings, 10k reports and press release operating in this market segment to fetch substantial information about the market size, trends, opportunity, drivers, restraints and to analyse key players and their market shares. Key companies are segmented at Tier level based on their revenues, product portfolio and presence.
Please note that these are the partial steps that are being followed while developing the market size. Besides this, forecasting will be done based on our internal proprietary model which also uses different macro-economic factors such as per capita healthcare expenditure, disposable income, industry based demand driving factors impacting the market and its forecast trends apart from disease related factors.
The report covers exhaustive analysis on:
By Technology
By End user
By Region






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Automated Feeding Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Cell Block Systems Market
Automated Compounding Systems Market
Automated Cell Culture Systems Market Analysis - Size, Share & Forecast 2025-2035
Automated Cell Biology Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Tool Grinding Systems Market Size and Share Forecast Outlook 2025 to 2035
Threat Detection Systems Market
Automated Colony Picking Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Sample Storage Systems Market Growth - Trends & Forecast 2025 to 2035
Automated Liquid Handling Systems Market
Automated Breast Ultrasound Systems Market Outlook - Share, Growth & Forecast 2025 to 2035
Automated Material Handling Systems Market - Market Outlook 2025 to 2035
Automated Microplate Handling Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Solid Phase Extraction Systems Market Size and Share Forecast Outlook 2025 to 2035
Automated Cell Therapy Processing Systems Market Trends - Outlook & Forecast 2025 to 2035
Automated Nucleic Acid Extraction Systems Market Analysis – Growth, Trends & Forecast 2024-2034
Wireless Fire Detection Systems Market Analysis - Size, Growth, and Forecast 2025 to 2035
Concealed Weapon Detection Systems Market Trends – Growth & Forecast 2025-2035
Automated Number Plate Recognition (ANPR) and Detection Sensors Market Size and Share Forecast Outlook 2025 to 2035
Automated Radionuclide Dispenser Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA