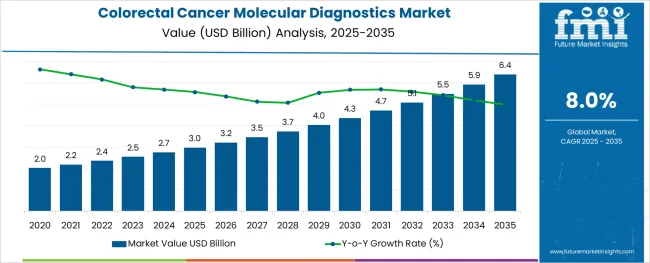
The Colorectal Cancer Molecular Diagnostics Market is estimated to be valued at USD 3.0 billion in 2025 and is projected to reach USD 6.4 billion by 2035, registering a compound annual growth rate (CAGR) of 8.0% over the forecast period.
The colorectal cancer molecular diagnostics market is expanding due to the increasing incidence of colorectal cancer worldwide and the rising emphasis on early and accurate diagnosis. Advances in molecular biology and genomics have enabled the development of highly sensitive diagnostic tools that detect genetic mutations and biomarkers associated with colorectal cancer.
The demand for personalized medicine has further accelerated adoption as these diagnostics guide targeted therapies and improve patient outcomes. Healthcare providers are investing in state-of-the-art diagnostic technologies to facilitate timely screening and disease monitoring.
Growing awareness about colorectal cancer screening programs and increasing government initiatives for cancer control are also supporting market growth. The integration of molecular diagnostics into routine clinical practice is expected to increase, enhancing disease detection and management. Segmental growth is anticipated to be driven by colorectal cancer molecular diagnostic reagents and kits, PCR technology, and hospitals as the primary end users due to their role in patient diagnostics and treatment planning.
The market is segmented by Product Type, Technology, and End User and region. By Product Type, the market is divided into Colorectal Cancer Molecular Diagnostic Reagents & Kits, Colorectal Cancer Molecular Diagnostic Instruments, and Services. In terms of Technology, the market is classified into PCR, Sequencing, Mass Spectrometry, Transcription Mediated Amplification, Chips and Microarrays, and Isothermal Nucleic Acid Amplification Technology. Based on End User, the market is segmented into Hospitals, Ambulatory Surgical Centers, Diagnostic Laboratories, and Homecare Settings. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
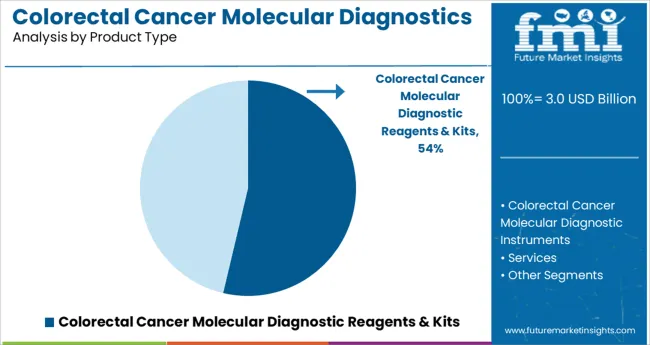
The colorectal cancer molecular diagnostic reagents and kits segment is expected to hold 53.7% of the market revenue in 2025, leading the product category. Growth in this segment is attributed to the essential role reagents and kits play in enabling accurate and efficient molecular testing. Laboratories and hospitals have adopted these kits for their standardized protocols and ease of use, allowing for consistent detection of cancer-specific genetic markers.
Continuous improvements in reagent formulations and assay sensitivity have enhanced diagnostic accuracy, which is critical for early-stage cancer detection. Furthermore, the availability of comprehensive kits covering a broad range of biomarkers has expanded their application in personalized oncology.
The segment’s dominance is also supported by increasing investments in molecular diagnostic infrastructure and growing demand for non-invasive testing options.
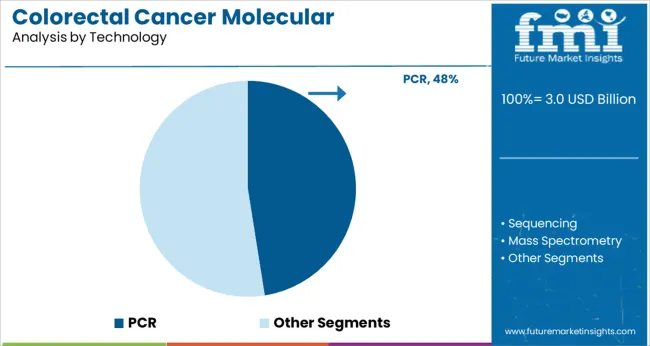
The PCR segment is projected to contribute 47.5% of the colorectal cancer molecular diagnostics market revenue in 2025, maintaining its position as the leading technology. PCR technology has been favored for its high sensitivity, specificity, and rapid turnaround time, making it suitable for detecting low-abundance genetic mutations in colorectal cancer.
It is widely used in clinical laboratories due to its reliability and well-established protocols. Advances in real-time PCR and multiplex PCR have further improved its application in cancer diagnostics by enabling simultaneous detection of multiple genetic targets.
The technology’s compatibility with automated platforms has facilitated high-throughput screening, supporting large-scale colorectal cancer screening programs. As molecular diagnostics evolve, PCR is expected to remain a foundational technology for colorectal cancer detection and monitoring.
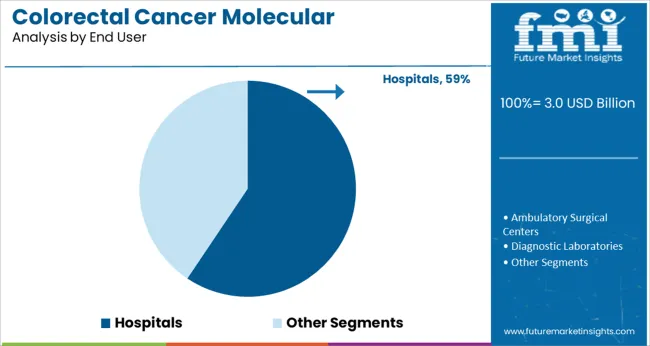
Hospitals are projected to account for 59.4% of the colorectal cancer molecular diagnostics market revenue in 2025, positioning them as the primary end users. This is driven by hospitals’ role as frontline healthcare providers responsible for patient diagnosis, treatment planning, and disease monitoring.
Hospitals have increasingly integrated molecular diagnostic testing into oncology departments and pathology labs to provide comprehensive cancer care. The availability of specialized equipment and trained personnel enables hospitals to perform advanced molecular assays and interpret complex results.
Additionally, hospital networks have adopted colorectal cancer screening protocols to improve early detection and reduce mortality rates. The growing collaboration between hospitals and diagnostic labs has expanded access to molecular testing services. With ongoing investments in healthcare infrastructure and cancer care programs, hospitals are expected to continue dominating the market as key users of colorectal cancer molecular diagnostics.
Increase in research in the pharmaceutical and biotechnology industry is contributing to the growth of the colorectal cancer molecular diagnostics market significantly. In addition, growing automation in laboratories is another factor supporting the growth of the colorectal cancer molecular diagnostics market.
Moreover, the ease of use of colorectal cancer molecular diagnostics, as well as accurate results is supporting the growth of the colorectal cancer molecular diagnostics market. All these factors are increasing the use of colorectal cancer molecular diagnostics.
Despite colorectal cancer being common across the globe, many people do not get themselves tested at an early stage. This is owing to the lack of knowledge regarding the problem and its lasting effects on health. In addition, the availability of low-cost alternatives and the high cost of instruments may hinder the growth of the market during the forecast period.
Limited specificity to some diagnostics tests that are unable to distinguish between inflammation and actual symptoms or cancer is another issue being faced by the colorectal cancer molecular diagnostics market.
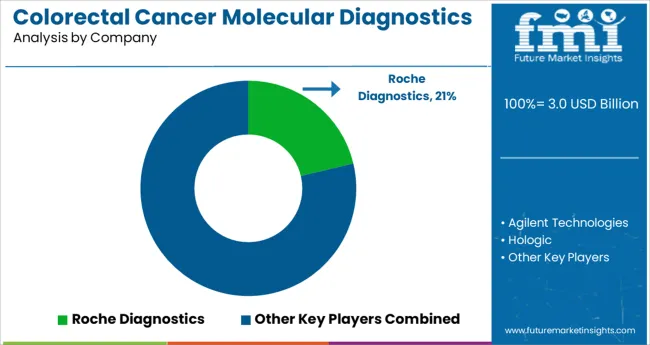
Some of the key players in the global Colorectal Cancer Molecular Diagnostics market are Dako, Gen Probe (Hologic), Cepheid, Qiagen, Roche Diagnostics, Bayer Healthcare, Abbott Laboratories, Grifols, Guardant Health, Danaher Corporation, Siemens, Beckton Dickinson, Biomérieux Sa, and Sysmex Corporation. Attributed to the presence of such high number of participants, the market is highly competitive.
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 8% from 2025 to 2035 |
| Market Value in 2025 | USD 2.35 Billion |
| Market Value in 2035 | USD 5.07 Billion |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2024 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in million and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends and Pricing Analysis |
| Segments Covered | Product Type, Technology, End User, Region |
| Regions Covered | North America; Latin America; Europe; Asia Pacific; Middle East and Africa |
| Key Countries Profiled | USA, Canada, Brazil, Mexico, Germany, UK, France, Spain, Italy, Nordics, BENELUX, Australia & New Zealand, China, India, ASEAN, GCC, South Africa |
| Key Companies Profiled | Dako; Gen Probe (Hologic); Cepheid Qiagen; Roche Diagnostics; Bayer Healthcare; Abbott Laboratories; Grifols, Danaher Corporation; Siemens; Beckton Dickinson; Biomérieux Sa ; Guardant Health; Sysmex Corporation |
| Customization | Available Upon Request |
The global colorectal cancer molecular diagnostics market is estimated to be valued at USD 3.0 billion in 2025.
It is projected to reach USD 6.4 billion by 2035.
The market is expected to grow at a 8.0% CAGR between 2025 and 2035.
The key product types are colorectal cancer molecular diagnostic reagents & kits, colorectal cancer molecular diagnostic instruments and services.
pcr segment is expected to dominate with a 47.5% industry share in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Cancer Registry Software Market Size and Share Forecast Outlook 2025 to 2035
Cancer Biological Therapy Market Size and Share Forecast Outlook 2025 to 2035
Cancer Biopsy Market - Growth & Technological Innovations 2025 to 2035
Cancer Vaccines Market Analysis by Technology, Treatment Method, Application and Region from 2025 to 2035
Cancer Gene Therapy Market Overview – Trends & Future Outlook 2024-2034
Cancer-focused Genetic Testing Service Market Analysis – Growth & Industry Insights 2024-2034
Cancer Tissue Diagnostic Market Trends – Growth & Industry Forecast 2024-2034
Cancer Supportive Care Products Market Trends – Growth & Forecast 2020-2030
Cancer Antigens Market
Cancer Diagnostics Market Analysis - Size, Share and Forecast 2025 to 2035
Pet Cancer Therapeutics Market Insights - Growth & Forecast 2024 to 2034
Skin Cancer Detection Devices Market Size and Share Forecast Outlook 2025 to 2035
Lung Cancer Surgery Market - Size, Share, and Forecast 2025 to 2035
Lung Cancer Therapeutics Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
Lung Cancer PCR Panel Market Trends, Growth, Demand & Forecast 2025 to 2035
Lung Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Blood Cancer Treatment Market Growth – Trends & Forecast 2025 to 2035
Brain Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Liver Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Canine Cancer Screening Services Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA