The high barrier pharmaceutical packaging films for blister market is forecast to experience steady growth, with a compound annual growth rate (CAGR) of 3.9% from 2025 to 2035. The market, valued at USD 6,003.5 million in 2025, is expected to reach USD 8,801.5 million by 2035, driven by increasing demand for high-quality, protective packaging solutions in the pharmaceutical industry. The need for pharmaceutical packaging films with high barrier properties has grown as the focus on patient safety, product integrity, and regulatory compliance intensifies. These films play a critical role in extending the shelf life of medications and maintaining their effectiveness, making them crucial in preventing moisture, oxygen, and light from affecting sensitive pharmaceutical products.
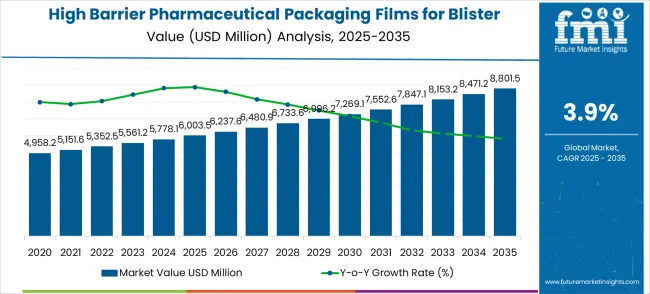
The market is likely to witness consistent growth as pharmaceutical companies seek more reliable and effective packaging solutions to meet the rising demand for oral solid dosage forms, particularly in emerging markets. As regulatory standards around drug safety and packaging evolve, manufacturers of blister packaging films will continue to innovate, developing products that enhance protection against external environmental factors. Despite the modest growth rate, the need for high barrier films in protecting the integrity of critical medicines, especially in blister packs, will keep the market resilient, ensuring that pharmaceutical packaging remains a vital segment within the broader pharmaceutical supply chain.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 6,003.5 million |
| Market Forecast Value (2035) | USD 8,801.5 million |
| Forecast CAGR (2025-2035) | 3.9% |
The high barrier pharmaceutical packaging films for blister market accounts for approximately 15% of the pharmaceutical packaging market, driven by the increasing demand for protective, tamper-proof, and moisture-resistant packaging solutions in the pharmaceutical industry. Within the flexible packaging market, it holds around 12%, reflecting its growing use in flexible, cost-efficient, and functional packaging designs. The barrier films market sees about 9%, as these films are crucial for maintaining the stability and efficacy of drugs. In the healthcare packaging market, the share is approximately 8%, as the demand for packaging solutions that preserve the integrity of pharmaceutical products continues to rise. Lastly, the blister packaging market captures about 10%, as high barrier films are a key component in modern blister packs. These contributions underscore the essential role of high barrier films in ensuring the safety, quality, and shelf-life of pharmaceutical products.
Market expansion is being supported by the rapid advancement of pharmaceutical manufacturing across developing economies and the corresponding need for protective packaging systems for drug stability and shelf life extension in diverse environmental conditions. Modern pharmaceutical operations require superior moisture and oxygen barrier properties to ensure optimal drug efficacy and regulatory compliance. The exceptional barrier performance and chemical resistance characteristics of high barrier pharmaceutical packaging films make them essential components in demanding pharmaceutical environments where product integrity is critical.
The growing emphasis on drug safety and regulatory compliance is driving demand for advanced packaging film technologies from certified manufacturers with proven track records of quality and regulatory approval. Pharmaceutical operators are increasingly investing in high-performance barrier systems that offer enhanced protection and extended shelf life over traditional packaging solutions. Regulatory standards and quality requirements are establishing performance benchmarks that favor precision-engineered high barrier packaging film solutions with advanced protective capabilities.
The market is segmented by material type, application, and region. By material type, the market is divided into PVC film, PVDC coated film, PP film, aluminum film, and other configurations. Based on application, the market is categorized into tablets, capsules, and other applications. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
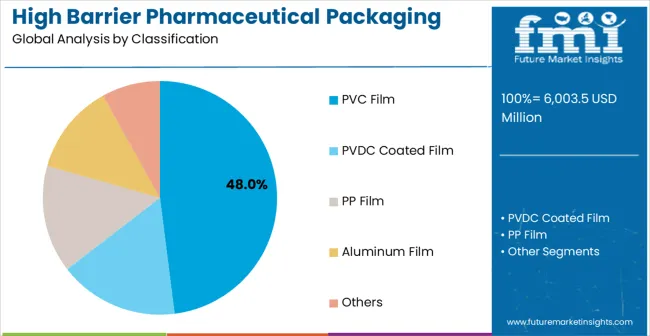
PVC film configurations are projected to account for 47% of the high barrier pharmaceutical packaging films for blister market in 2025. This leading share is supported by the increasing demand for cost-effective barrier solutions in pharmaceutical packaging applications and growing requirements for versatile processing characteristics. PVC films provide excellent thermoforming properties and chemical resistance, making them the preferred choice for tablet packaging, generic drug applications, and standard pharmaceutical blister packs. The segment benefits from technological advancements that have improved the barrier properties and processing efficiency of PVC film formulations while maintaining cost-effectiveness and regulatory compliance.
Modern PVC pharmaceutical films incorporate advanced polymer formulations, barrier coatings, and surface treatments that enhance moisture protection and extend drug shelf life. These innovations have significantly improved pharmaceutical packaging performance while reducing total cost of ownership through enhanced processability and elimination of packaging failures. The generic pharmaceuticals and over-the-counter drug sectors particularly drive demand for PVC film solutions, as these industries require cost-effective protection to maintain drug quality and comply with regulatory standards.
Additionally, the emerging market pharmaceutical industry increasingly adopts PVC films to balance protection requirements with cost considerations and meet international packaging standards. Market accessibility requirements and regulatory compliance initiatives further accelerate market adoption, as PVC systems provide reliable protection while maintaining competitive pricing and processing efficiency.
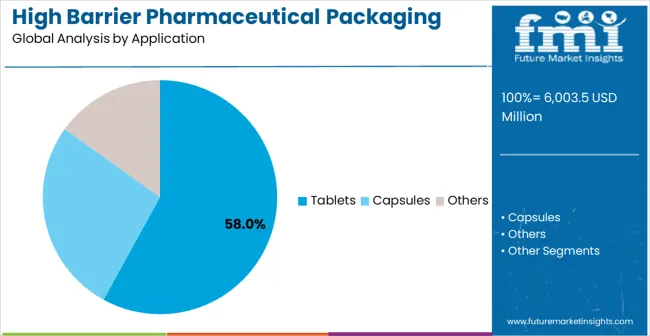
Tablets applications are expected to represent 58% of high barrier pharmaceutical packaging films for blister demand in 2025. This dominant share reflects the critical role of blister packaging in tablet protection and the need for reliable barrier solutions capable of maintaining drug stability in diverse storage and distribution conditions. Tablet manufacturers require effective and durable packaging films for moisture protection, oxygen barrier, and tamper evidence. The segment benefits from ongoing expansion of oral solid dosage form production in developing countries and increasing quality requirements demanding enhanced barrier systems in pharmaceutical manufacturing facilities.
Tablet packaging applications demand exceptional barrier performance to ensure drug efficacy and shelf life stability. These applications require films capable of handling various environmental conditions, storage requirements, and distribution challenges while maintaining drug integrity throughout the product lifecycle. The growing emphasis on pharmaceutical quality standards, particularly in generic drug manufacturing and international market distribution, drives consistent demand for high-performance barrier film solutions. Emerging pharmaceutical markets in Asia-Pacific, Latin America, and European regions contribute significantly to market growth as drug manufacturers invest in modern packaging technologies to improve product quality and market competitiveness.
Additionally, the trend toward patient-friendly packaging and unit-dose applications creates opportunities for specialized barrier film systems equipped with enhanced protective capabilities and user convenience features. The segment also benefits from increasing chronic disease medication production and aging population demographics driven by growing demand for long-term medication storage and patient compliance solutions.
The high barrier pharmaceutical packaging films for blister market is advancing steadily due to increasing pharmaceutical production and growing recognition of drug protection technology importance. However, the market faces challenges including regulatory compliance complexity, need for specialized processing expertise, and varying barrier requirements across different pharmaceutical applications. Standardization efforts and certification programs continue to influence film quality and market development patterns.
The growing deployment of intelligent packaging systems and digital authentication capabilities is enabling real-time drug monitoring and anti-counterfeiting protection capabilities in pharmaceutical blister packaging. Smart sensors and tamper-evident technologies provide continuous product verification while enhancing supply chain security and extending drug traceability. These technologies are particularly valuable for high-value pharmaceuticals that require comprehensive protection against counterfeiting and supply chain integrity monitoring.
Modern pharmaceutical film manufacturers are incorporating recyclable materials and bio-based barrier layers that improve environmental sustainability while maintaining protective performance through advanced material formulations and coating technologies. Integration of renewable raw materials and reduced environmental impact enables superior sustainability credentials and significant environmental benefits compared to traditional petroleum-based packaging films. Advanced manufacturing processes and quality control systems also support development of more consistent and reliable pharmaceutical packaging films for demanding regulatory environments.
| Country | CAGR (2025-2035) |
|---|---|
| China | 5.3% |
| India | 4.9% |
| Germany | 4.5% |
| Brazil | 4.1% |
| United States | 3.7% |
| United Kingdom | 3.3% |
| Japan | 2.9% |
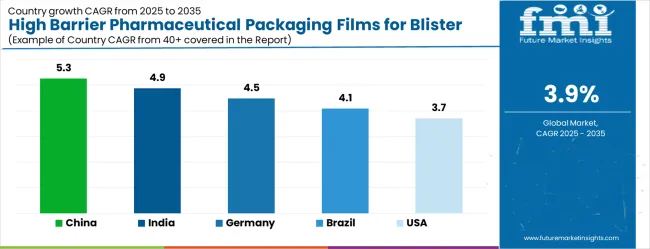
The high barrier pharmaceutical packaging films for blister market is growing steadily, with China leading at a 5.3% CAGR through 2035, driven by massive pharmaceutical manufacturing expansion, generic drug production growth, and domestic pharmaceutical market development. India follows at 4.9%, supported by rising pharmaceutical investments and increasing adoption of advanced packaging technologies in drug manufacturing and export operations. Germany records strong growth at 4.5%, emphasizing precision engineering, pharmaceutical excellence, and advanced packaging technology capabilities. Brazil grows steadily at 4.1%, integrating high barrier packaging films into expanding pharmaceutical production and healthcare infrastructure facilities. The United States shows moderate growth at 3.7%, focusing on pharmaceutical innovation and packaging technology advancement improvements. The United Kingdom maintains steady expansion at 3.3%, supported by pharmaceutical manufacturing programs. Japan demonstrates stable growth at 2.9%, emphasizing technological innovation and pharmaceutical packaging excellence.
The report covers an in-depth analysis of 40+ countries, the top-performing countries are highlighted below.
The high barrier pharmaceutical packaging films for blister market in China is projected to exhibit the highest growth rate with a CAGR of 5.3% through 2035, driven by rapid pharmaceutical manufacturing expansion and massive generic drug production development programs across pharmaceutical sectors. The country's growing API manufacturing and expanding pharmaceutical export industries are creating significant demand for high-performance packaging film systems. Major pharmaceutical companies are establishing comprehensive packaging installations to support large-scale drug production operations and meet international quality standards.
China's pharmaceutical sector continues expanding rapidly, with major investments in generic drug manufacturing, API production, and pharmaceutical export operations. The country's position as a global pharmaceutical manufacturing hub drives substantial demand for reliable packaging film solutions that ensure drug quality and regulatory compliance across diverse pharmaceutical applications.
The high barrier pharmaceutical packaging films for blister market in India is expanding at a CAGR of 4.9%, supported by increasing pharmaceutical investments across manufacturing sectors and growing adoption of advanced packaging technologies in drug manufacturing and export operations. The country's expanding generic drug industry is driving demand for specialized packaging systems capable of handling diverse regulatory and quality requirements. Pharmaceutical facilities are investing in advanced barrier technologies to improve product quality and comply with international standards.
India's pharmaceutical development initiatives and export expansion create significant opportunities for high barrier packaging film applications. The country's growing pharmaceutical market drives demand for generic drugs and API production, requiring reliable packaging solutions to maintain drug quality and market competitiveness.
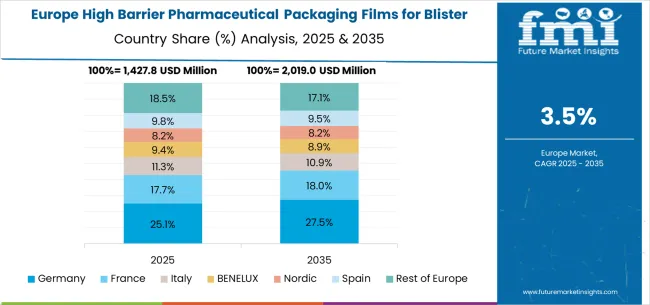
The high barrier pharmaceutical packaging films for blister market in Germany is projected to grow at a CAGR of 4.5%, supported by the country's emphasis on pharmaceutical excellence and advanced packaging technology capabilities. German pharmaceutical facilities are implementing high-performance packaging systems that meet stringent quality standards and regulatory reliability requirements. The market is characterized by focus on technological innovation, advanced materials, and compliance with comprehensive pharmaceutical regulations.
Germany's advanced pharmaceutical sector and packaging excellence capabilities drive demand for high-quality barrier film solutions. The country's emphasis on pharmaceutical innovation, technological advancement, and quality precision creates opportunities for advanced packaging technologies that meet demanding pharmaceutical specifications.
The high barrier pharmaceutical packaging films for blister market in Brazil is growing at a CAGR of 4.1%, driven by expanding pharmaceutical production and increasing healthcare infrastructure development across pharmaceutical sectors. The country's growing generic drug manufacturing is investing in advanced packaging systems to improve drug quality and performance in competitive market conditions. Pharmaceutical facilities are adopting modern barrier technologies to support growing production requirements and quality standards.
Brazil's expanding pharmaceutical infrastructure and healthcare development create substantial opportunities for packaging film applications. The country's growing pharmaceutical market and domestic production expansion drive investments in drug protection solutions that enhance product competitiveness and operational efficiency.
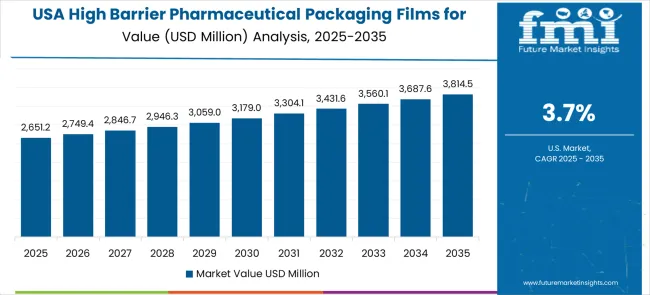
The high barrier pharmaceutical packaging films for blister market in the United States is expanding at a CAGR of 3.7%, driven by ongoing pharmaceutical innovation and increasing emphasis on packaging technology advancement solutions. Pharmaceutical facilities are upgrading existing packaging systems with advanced technologies that provide improved performance and enhanced drug protection capabilities. The market benefits from replacement demand and facility modernization programs across multiple pharmaceutical sectors.
The United States market emphasizes pharmaceutical innovation, technology advancement, and packaging improvement in barrier film applications. Advanced research and development activities drive the adoption of next-generation packaging technologies that offer superior performance and regulatory compliance capabilities.
The high barrier pharmaceutical packaging films for blister market in the United Kingdom is projected to grow at a CAGR of 3.3%, supported by ongoing pharmaceutical manufacturing programs and packaging technology facility upgrades. Pharmaceutical operators are investing in reliable barrier film systems that provide consistent performance and meet regulatory compliance requirements. The market is characterized by focus on system reliability, operational efficiency, and pharmaceutical compliance across diverse drug manufacturing applications.
The United Kingdom's focus on pharmaceutical manufacturing and packaging excellence drives demand for high-performance barrier film solutions. The country's emphasis on drug quality, manufacturing efficiency, and pharmaceutical compliance creates opportunities for advanced packaging technologies.
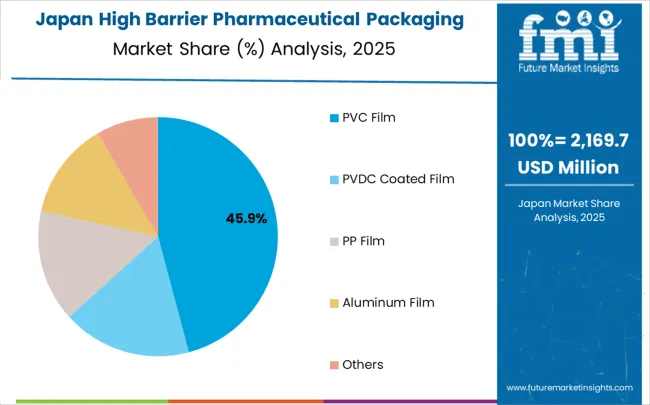
The high barrier pharmaceutical packaging films for blister market in Japan is expanding at a CAGR of 2.9%, driven by the country's emphasis on technological innovation and pharmaceutical packaging excellence. Japanese manufacturers are developing advanced packaging film technologies that incorporate precision engineering and efficiency-optimized design principles. The market benefits from focus on quality, reliability, and continuous improvement in pharmaceutical packaging technology performance.
Japan's technological leadership and pharmaceutical expertise drive the development of advanced barrier film solutions. The country's emphasis on innovation, quality control, and packaging optimization creates opportunities for cutting-edge packaging technologies that set pharmaceutical industry standards.
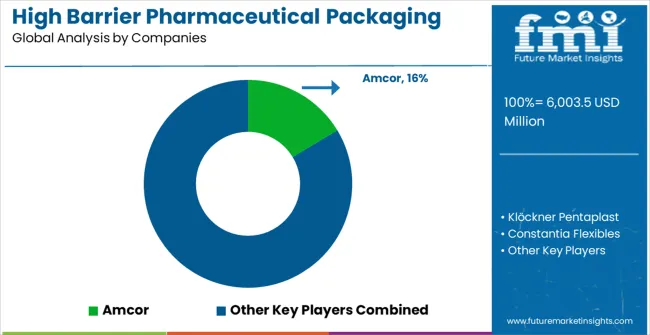
The high barrier pharmaceutical packaging films for blister market is defined by competition among established packaging materials manufacturers, specialized pharmaceutical packaging companies, and emerging sustainable packaging solution providers. Companies are investing in advanced barrier technologies, regulatory compliance capabilities, standardized quality systems, and technical support services to deliver reliable, compliant, and cost-effective pharmaceutical packaging solutions. Strategic partnerships, technological advancement, and geographic expansion are central to strengthening product portfolios and market presence.
Amcor, operating globally, offers comprehensive pharmaceutical packaging solutions with focus on barrier excellence, regulatory compliance, and technical support services. Klöckner Pentaplast, multinational manufacturer, provides advanced packaging film systems with emphasis on pharmaceutical applications and regulatory integration capabilities. Constantia Flexibles, specialized provider, delivers innovative barrier packaging solutions for pharmaceutical applications with focus on quality and performance. Perlen Packaging (CPH) offers comprehensive packaging technologies with standardized procedures and pharmaceutical service support.
Tekni-plex provides advanced pharmaceutical packaging systems with emphasis on specialty applications and technical expertise. Honeywell, Liveo Research GmbH deliver specialized barrier solutions with focus on high-performance pharmaceutical applications. Sumitomo Bakelite, HySum, Aluberg offer comprehensive pharmaceutical packaging technologies for demanding regulatory environments with established manufacturing and service capabilities.
Bilcare, SÜDPACK, FlexiPack, Etimex Primary Packaging, Uniworth, Sichuan Huili Industry, Jiangxi Chunguang New Materials, Hangzhou Plastics Industry, Jiangsu Fuxin, Huakang Packaging, LIAONING TOTEM PACKAGING provide advanced packaging film systems with regional manufacturing capabilities, pharmaceutical industry expertise, and specialized knowledge across packaging technology and pharmaceutical compliance sectors.
The high barrier pharmaceutical packaging films for blister market underpins pharmaceutical quality excellence, drug safety advancement, regulatory compliance optimization, and patient health protection. With drug safety mandates, stricter regulatory requirements, and demand for protective packaging systems, the sector must balance cost competitiveness, barrier performance, and regulatory compliance. Coordinated contributions from governments, industry bodies, OEMs/technology integrators, suppliers, and investors will accelerate the transition toward performance-optimized, regulatory-compliant, and technologically advanced pharmaceutical packaging systems.
| Item | Value |
|---|---|
| Quantitative Units | USD 6,003.5 million |
| Material Type | PVC Film, PVDC Coated Film, PP Film, Aluminum Film, Others |
| Application | Tablets, Capsules, Others |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Germany, India, China, United Kingdom, Japan, Brazil, and other 40+ countries |
| Key Companies Profiled | Amcor, Klöckner Pentaplast, Constantia Flexibles, Perlen Packaging (CPH), Tekni-plex, Honeywell, Liveo Research GmbH, Sumitomo Bakelite, HySum, Aluberg, Bilcare, SÜDPACK, FlexiPack, Etimex Primary Packaging, Uniworth, Sichuan Huili Industry, Jiangxi Chunguang New Materials, Hangzhou Plastics Industry, Jiangsu Fuxin, Huakang Packaging, LIAONING TOTEM PACKAGING |
| Additional Attributes | Dollar sales by material type and application segments, regional demand trends across North America, Europe, and Asia-Pacific, competitive landscape with established manufacturers and emerging suppliers, buyer preferences for high-barrier versus cost-effective packaging systems, integration with pharmaceutical manufacturing and regulatory compliance technologies, innovations in barrier technology and sustainable materials for enhanced drug protection and environmental sustainability, and adoption of smart packaging solutions with embedded track-and-trace and tamper-evidence capabilities for improved pharmaceutical safety and regulatory compliance. |
The global high barrier pharmaceutical packaging films for blister market is estimated to be valued at USD 6,003.5 million in 2025.
The market size for the high barrier pharmaceutical packaging films for blister market is projected to reach USD 8,801.5 million by 2035.
The high barrier pharmaceutical packaging films for blister market is expected to grow at a 3.9% CAGR between 2025 and 2035.
The key product types in high barrier pharmaceutical packaging films for blister market are pvc film, pvdc coated film, pp film, aluminum film and others.
In terms of application, tablets segment to command 58.0% share in the high barrier pharmaceutical packaging films for blister market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
High Protein Powders Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
High Purity Gas Flow Meter Market Size and Share Forecast Outlook 2025 to 2035
High Purity Flow Meter Market Size and Share Forecast Outlook 2025 to 2035
High Airtight Storage Cabinets Market Size and Share Forecast Outlook 2025 to 2035
High Voltage Porcelain Bushing Market Size and Share Forecast Outlook 2025 to 2035
High Octane Racing Fuel Market Size and Share Forecast Outlook 2025 to 2035
High Voltage Air-cooled Battery Compartment Market Size and Share Forecast Outlook 2025 to 2035
High Temperature NiMH Battery Market Size and Share Forecast Outlook 2025 to 2035
High Voltage Cable Termination Market Size and Share Forecast Outlook 2025 to 2035
High Security Wedge Barricades Market Size and Share Forecast Outlook 2025 to 2035
High Purity Chemical Filters Market Size and Share Forecast Outlook 2025 to 2035
High-vacuum Fiber Feedthrough Flanges Market Size and Share Forecast Outlook 2025 to 2035
High Pressure Grease Hose Market Size and Share Forecast Outlook 2025 to 2035
High Reliability Oscillators Market Size and Share Forecast Outlook 2025 to 2035
High Purity Magnesium Citrate Market Size and Share Forecast Outlook 2025 to 2035
High-frequency RF Evaluation Board Market Size and Share Forecast Outlook 2025 to 2035
High Viscosity Mixer Market Size and Share Forecast Outlook 2025 to 2035
High Voltage Ionising Air Gun Market Size and Share Forecast Outlook 2025 to 2035
High Voltage Equipment Market Forecast and Outlook 2025 to 2035
High Clear Film Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA