The demand for 1,8-dinitroanthraquinone in the USA is forecast to grow from USD 1.5 million in 2025 to approximately USD 2.0 million by 2035, recording an absolute increase of USD 0.5 million over the forecast period. This translates into total growth of 33.3%, with demand forecast to expand at a compound annual growth rate (CAGR) of 2.9% between 2025 and 2035. Expansion is supported by wider usage of high-grade intermediates required for synthesis efficiency, pharmaceutical formulation accuracy, and chemical processing reliability across major research and production corridors. Purity ≥98% holds a 64.8% share in 2025, favored for compatibility with existing equipment and its stable performance profile across pharmaceutical workflows.
Purity ≥99% gains interest among facilities seeking enhanced molecular precision for advanced synthesis. Pharmaceutical manufacturing accounts for 52.7% of demand due to its reliance on controlled-purity compounds that support yield stability, process safety, and predictable synthesis pathways. Dye production and broader chemical processing account for the remaining share as specialty compound needs evolve across regional manufacturing clusters. The Northeast leads with a 3.2% CAGR, supported by concentrated research facilities and a strong pharmaceutical presence, while the West follows at 3.0% through biotechnology density and chemical capabilities. The South at 2.8% and the Midwest at 2.6% sustain adoption by expanding chemical sites and modernizing regional pharmaceutical operations.
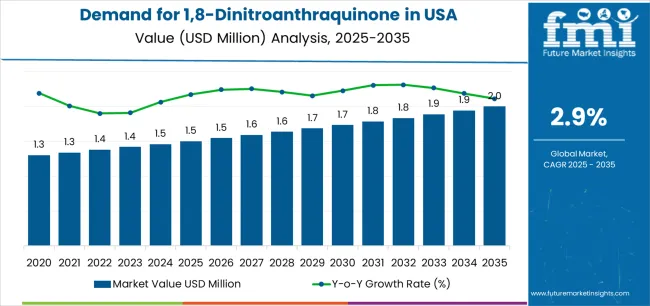
From 2030 to 2035, demand is forecast to grow from USD 1.7 million to USD 2.0 million, adding another USD 0.3 million, which constitutes 60.0% of the overall ten-year expansion. This period is expected to be characterized by expansion of pharmaceutical synthesis technologies, integration of specialized chemical processing systems and quality management networks, and development of advanced intermediate pathways across different pharmaceutical applications. The growing adoption of precision synthesis principles and enhanced quality control requirements, particularly in Northeast and West regions, will drive demand for more sophisticated chemical systems and specialized purity specifications.
Between 2020 and 2025, USA 1,8-dinitroanthraquinone demand experienced steady expansion, driven by increasing pharmaceutical operational requirements in specialty chemical sectors and growing awareness of high-purity compound benefits for synthesis enhancement and operational reliability. The sector developed as pharmaceutical manufacturers and chemical companies, especially in major research corridors, recognized the need for specialized intermediates and reliable quality management to achieve synthesis targets while meeting pharmaceutical expectations and efficiency requirements. Chemical suppliers and pharmaceutical manufacturers began emphasizing proper performance optimization and application integration to maintain synthesis effectiveness and commercial viability.
| Metric | Value |
|---|---|
| USA 1,8-Dinitroanthraquinone Sales Value (2025) | USD 1.5 million |
| USA 1,8-Dinitroanthraquinone Forecast Value (2035) | USD 2.0 million |
| USA 1,8-Dinitroanthraquinone Forecast CAGR (2025-2035) | 2.9% |
Demand expansion is being supported by the accelerating emphasis on pharmaceutical manufacturing excellence and chemical processing optimization nationwide, with the USA maintaining its position as a pharmaceutical and chemical synthesis innovation leadership region, and the corresponding need for effective high-purity intermediate systems for drug development, synthesis efficiency, and quality assurance integration. Modern pharmaceutical strategies rely on 1,8-dinitroanthraquinone technologies to ensure synthesis competitiveness, quality compliance, and optimal pathway achievement toward efficiency-focused pharmaceutical operations. Chemical operational requirements necessitate comprehensive intermediate solutions including advanced purity processing, molecular management capabilities, and performance control infrastructure to address diverse pharmaceutical needs and synthesis specifications.
The growing emphasis on pharmaceutical quality standards and increasing federal and state-level chemical regulations, particularly synthesis excellence commitments across the USA, are driving demand for high-purity intermediate systems from proven chemical suppliers with appropriate pharmaceutical expertise and quality management capabilities. Pharmaceutical manufacturers and chemical companies are increasingly investing in specialized compound sourcing and integrated synthesis solutions to enhance pharmaceutical profiles, access efficiency trends, and demonstrate chemical leadership in competitive pharmaceutical environments. Chemical policies and quality control requirements are establishing standardized intermediate pathways that require high-purity systems and performance assurance, with USA pharmaceutical operations often pioneering large-scale implementation of specialized chemical technologies.
Demand is segmented by purity grade, application, and region. By purity grade, sales are divided into purity ≥98%, purity ≥99%, and other purity configurations. In terms of application, sales are segmented into pharmaceutical manufacturing, dye production, chemical processing, and others. Regionally, demand is divided into Northeast, West, South, and Midwest, with Northeast representing a key growth and innovation hub for 1,8-dinitroanthraquinone technologies.
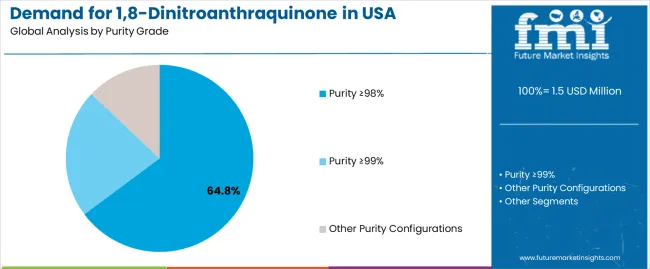
The purity ≥98% segment is projected to account for 64.8% of USA 1,8-dinitroanthraquinone demand in 2025, making it the leading purity category across the sector. This dominance reflects the operational balance and quality suitability of high-grade chemical systems for existing pharmaceutical facilities and chemical applications where synthesis performance is optimized through controlled purity specifications. In the USA, where substantial pharmaceutical infrastructure requires intermediate integration without complete equipment replacement, purity ≥98% systems provide practical pathways for synthesis enhancement while maintaining operational efficiency continuity. Continuous innovations are improving chemical effectiveness, molecular stability characteristics, and application compatibility parameters, enabling manufacturers to achieve high performance standards while minimizing operational cost increases. The segment's strong position is reinforced by the extensive existing pharmaceutical infrastructure requiring intermediate adoption and growing availability of purity ≥98% technology suppliers with proven commercial experience.
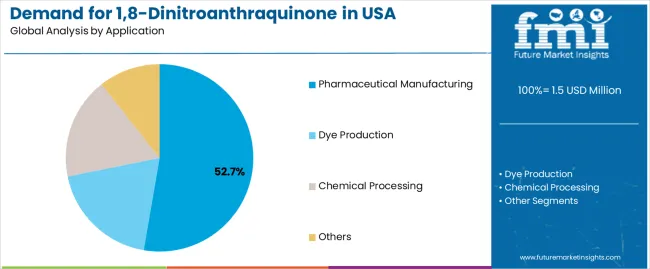
Pharmaceutical manufacturing applications are expected to represent 52.7% of USA 1,8-dinitroanthraquinone demand in 2025, highlighting the critical importance of drug development requiring specialized chemical solutions. Pharmaceutical facilities including research laboratories, production operations, specialty synthesis, and pharmaceutical manufacturing applications generate consistent demand for chemical systems that are technically and economically favorable for high-purity applications. The segment benefits from synthesis characteristics that often provide superior operational reliability compared to standard alternatives, reducing complexity and costs. Pharmaceutical applications also access enhanced efficiency through specialized compounds that improve synthesis performance and appeal. In the USA, where pharmaceutical innovation represents substantial portions of chemical development, specialized compound deployment requires 1,8-dinitroanthraquinone integration across diverse pharmaceutical operations. In Northeast and West regions, where pharmaceutical concentrations are significant, 1,8-dinitroanthraquinone demand is elevated by emphasis on maintaining synthesis efficiency while achieving pharmaceutical integration targets.
USA 1,8-dinitroanthraquinone demand is advancing steadily due to increasing pharmaceutical efficiency and growing recognition of specialized chemical necessity for pharmaceutical development, with Northeast region serving as a key driver of innovation and application development. The sector faces challenges including competition from alternative compounds, purity cost considerations, and ongoing concerns regarding initial investment costs and supply chain requirements. Federal pharmaceutical guidelines and state-level quality initiatives, particularly chemical programs in Northeast and West regions, continue to influence compound selection and deployment timelines.
The enhancement of pharmaceutical quality regulations, gaining particular significance through drug development guidelines and synthesis excellence campaigns, is enabling chemical suppliers to achieve differentiation without prohibitive investment costs, providing predictable demand patterns through pharmaceutical requirements and synthesis efficiency preferences. Enhanced quality standards offering substantial opportunities for high-purity chemical systems and pharmaceutical applications provide foundational dynamics while allowing suppliers to secure pharmaceutical agreements and synthesis partnerships. These trends are particularly valuable for first-mover suppliers and premium chemical development that require substantial purity investments without immediate cost advantages.
Modern chemical suppliers and manufacturers are establishing advanced quality management networks and centralized synthesis monitoring facilities that improve operational efficiency through purity standardization and economies of scale. Integration of specialized chemical systems, real-time quality monitoring, and coordinated synthesis management enables more efficient compound operation across multiple pharmaceutical sources. Advanced chemical concepts also support next-generation pharmaceutical applications including specialized facility integration, synthesis cluster optimization, and regional chemical supply networks that optimize system-level economics while enabling comprehensive efficiency across pharmaceutical regions, with USA developments increasingly adopting collaborative chemical models to reduce individual operator costs and accelerate deployment.
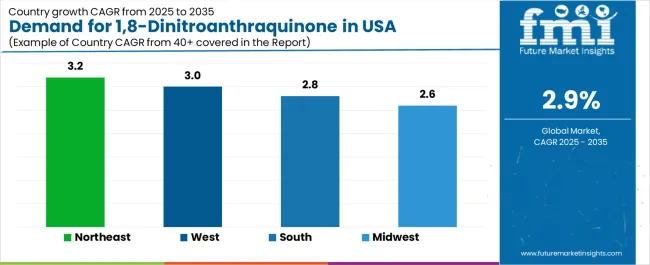
| Region | CAGR (2025-2035) |
|---|---|
| Northeast | 3.2% |
| West | 3.0% |
| South | 2.8% |
| Midwest | 2.6% |
The USA 1,8-dinitroanthraquinone demand is witnessing consistent growth, supported by rising pharmaceutical efficiency, expanding quality requirements, and the deployment of advanced chemical technologies across regions. Northeast leads the nation with a 3.2% CAGR, reflecting progressive pharmaceutical trends, substantial research innovation, and early adoption of premium chemical systems. West follows with a 3.0% CAGR, driven by extensive pharmaceutical infrastructure, favorable research demographics, and concentration of chemical operations that enhance application development. South grows at 2.8%, as pharmaceutical modernization and synthesis efficiency opportunities increasingly drive compound deployment. Midwest demonstrates growth at 2.6%, supported by expanding chemical facilities and regional pharmaceutical initiatives.
Why Does the Northeast Lead USA 1,8-Dinitroanthraquinone Growth with a 3.2% CAGR?
Demand for 1,8-dinitroanthraquinone in Northeast is projected to exhibit exceptional growth with a CAGR of 3.2% through 2035, driven by progressive pharmaceutical efficiency preferences, substantial research development creating premium chemical opportunities, and concentration of innovation across Massachusetts and surrounding states. As the dominant region with extensive pharmaceutical infrastructure and efficiency-focused operational policies, Northeast's emphasis on comprehensive drug development and chemical leadership is creating significant demand for advanced 1,8-dinitroanthraquinone systems with proven performance and reliable application potential. Major pharmaceutical manufacturers and chemical suppliers are establishing comprehensive compound development programs to support research innovation and premium chemical deployment across diverse applications.
How Is the West Leveraging Its Pharmaceutical Infrastructure to Strengthen Demand?
Demand for 1,8-dinitroanthraquinone in West is expanding at a CAGR of 3.0%, supported by extensive pharmaceutical facilities including biotechnology companies, research operations, and chemical establishments generating concentrated demand favorable for high-purity chemical systems. The region's operational characteristics, featuring substantial pharmaceutical operations and synthesis efficiency requirements ideal for specialized integration, provide natural advantages. Pharmaceutical industry expertise concentrated in California, Washington, and regional research corridors facilitates application development and operational management. Chemical suppliers and manufacturers are implementing comprehensive compound strategies to serve expanding efficiency-focused requirements throughout West.
Why Is the South Sustaining Steady Growth Through Chemical Management Efficiency?
Demand for 1,8-dinitroanthraquinone in South is growing at a CAGR of 2.8%, driven by substantial chemical management facilities from pharmaceutical operations, manufacturing services, and regional processing requiring high-purity compound pathways. The region's pharmaceutical base, supporting critical chemical operations, is increasingly adopting compound technologies to maintain competitiveness while meeting efficiency expectations. Manufacturers and chemical suppliers are investing in compound integration systems and regional supply infrastructure to address growing quality requirements.
How Is the Midwest Expanding 1,8-Dinitroanthraquinone Adoption Through Facility Development?
Demand for 1,8-dinitroanthraquinone in Midwest is advancing at a CAGR of 2.6%, supported by expanding chemical facilities, regional pharmaceutical development including specialized processing applications, and growing emphasis on compound solutions across the region. Chemical modernization and pharmaceutical facility expansion are driving consideration of high-purity compounds as operational enhancement pathways. Pharmaceutical companies and chemical suppliers are developing regional capabilities to support emerging compound deployment requirements.
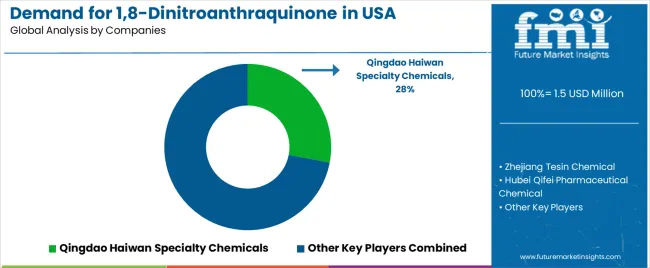
Demand for 1,8-dinitroanthraquinone in the United States is defined by competition among specialized chemical manufacturers, pharmaceutical companies, and synthesis solution providers, with major chemical corporations maintaining significant influence through supply chain resources and technology development capabilities. Companies are investing in purity technology advancement, molecular optimization, distribution network structures, and comprehensive application services to deliver effective, reliable, and scalable chemical solutions across the USA pharmaceutical and chemical applications. Strategic partnerships, technology infrastructure development, and first-mover application execution are central to strengthening competitive positioning and presence across pharmaceutical applications, research facilities, and synthesis applications.
Qingdao Haiwan Specialty Chemicals, an internationally recognized chemical leader, leads with a 28% share, offering a comprehensive supply of 1,8-dinitroanthraquinone, including manufacturing, technology, and distribution services, with a focus on pharmaceutical applications, performance reliability, and cost optimization across USA operations. Zhejiang Tesin Chemical, operating nationally with extensive USA distribution, provides integrated chemical solutions leveraging engineering expertise, quality assurance development, and large-scale manufacturing capabilities.
Hubei Qifei Pharmaceutical Chemical delivers full-service 1,8-dinitroanthraquinone processing, including synthesis technology, performance testing, and supply management, serving USA and international pharmaceutical projects. Shengao Chemical Industry emphasizes comprehensive specialty chemical solutions with integrated technology, quality control, and distribution capabilities, leveraging pharmaceutical sector expertise. Jiangsu Yabang Dyestuff provides 1,8-dinitroanthraquinone application development and quality assurance services for pharmaceutical and chemical applications across the USA.
1,8-Dinitroanthraquinone represents critical chemical infrastructure for enhancing pharmaceutical productivity, supporting synthesis efficiency, and enabling quality applications essential for achieving pharmaceutical performance targets. With the demand projected to reach USD 2.0 million by 2035, driven by pharmaceutical efficiency, quality requirements, and technology advancement, the sector stands at the intersection of pharmaceutical innovation, synthesis excellence, and chemical development. The compound ecosystem spanning synthesis systems, supply chain networks, quality monitoring facilities, and application development infrastructure requires coordinated action across chemical suppliers, manufacturers, pharmaceutical distributors, regulatory authorities, research institutions, and pharmaceutical organizations to unlock its full value potential while addressing the technical complexities of large-scale chemical management and consistent performance delivery.
How Governments Could Accelerate Development and Performance Standards?
How Industry Bodies Could Strengthen Sector Development?
How Chemical Suppliers Could Capture Value and Drive Innovation?
How Pharmaceutical Manufacturers Could Optimize Chemical Enhancement Strategies?
How Research Companies Could Lead Chemical Integration?
How Biotechnology Companies Could Unlock Pharmaceutical Innovation?
How Investors and Financial Enablers Could Unlock Growth?
| Item | Value |
|---|---|
| Quantitative Units | USD 2.0 million |
| Purity Grade | Purity ≥98%, Purity ≥99%, Other Purity Configurations |
| Application | Pharmaceutical manufacturing, dye production, chemical processing, others |
| Regions Covered | Northeast, West, South, Midwest |
| Key Companies Profiled | Qingdao Haiwan Specialty Chemicals, Zhejiang Tesin Chemical, Hubei Qifei Pharmaceutical Chemical, Shengao Chemical Industry |
| Additional Attributes | Sales by purity grade and application segment, regional demand trends across Northeast, West, South, and Midwest, competitive landscape with established chemical suppliers and specialized pharmaceutical manufacturers, manufacturer preferences for purity ≥98% versus other chemical technologies, integration with pharmaceutical efficiency programs and quality policies particularly advanced in Northeast region, innovations in compound efficiency and performance enhancement technologies |
The global demand for 1,8-dinitroanthraquinone in USA is estimated to be valued at USD 1.5 million in 2025.
The market size for the demand for 1,8-dinitroanthraquinone in USA is projected to reach USD 2.0 million by 2035.
The demand for 1,8-dinitroanthraquinone in USA is expected to grow at a 2.9% CAGR between 2025 and 2035.
The key product types in demand for 1,8-dinitroanthraquinone in USA are purity ≥98%, purity ≥99% and other purity configurations.
In terms of application, pharmaceutical manufacturing segment to command 52.7% share in the demand for 1,8-dinitroanthraquinone in USA in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Demand Signal Repository Solutions Market Size and Share Forecast Outlook 2025 to 2035
Demand Side Management Market Size and Share Forecast Outlook 2025 to 2035
Demand Response Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
North America Shipping Supplies Market Trends – Innovations & Growth 2024-2034
Demand of Kozani Saffron in Greece Analysis - Size, Share & Forecast 2025 to 2035
Demand of No-acid Whey Strained Dairy Processing Concepts in European Union Size and Share Forecast Outlook 2025 to 2035
Demand for Bronte Pistachio in Italy Analysis - Size, Share & Forecast 2025 to 2035
Demand and Trend Analysis of Gaming Monitor in Western Europe Size and Share Forecast Outlook 2025 to 2035
Demand and Trend Analysis of Gaming Monitor in Japan Size and Share Forecast Outlook 2025 to 2035
Demand and Trend Analysis of Gaming Monitor in Korea Size and Share Forecast Outlook 2025 to 2035
Glycine Soja (Soybean) Seed Extract Market Size and Share Forecast Outlook 2025 to 2035
Demand and Trend Analysis of Yeast in Japan - Size, Share, and Forecast Outlook 2025 to 2035
Demand and Trends Analysis of Stevia in Japan Size and Share Forecast Outlook 2025 to 2035
Demand of Pistachio-based desserts & ingredients in France Analysis - Size, Share & Forecast 2025 to 2035
Japan Women’s Intimate Care Market Trends – Growth & Forecast 2024-2034
Western Europe Men’s Skincare Market Analysis – Forecast 2023-2033
Demand and Trend Analysis of Fabric Stain Remover in Korea Size and Share Forecast Outlook 2025 to 2035
Demand and Sales Analysis of Paper Cup in Japan Size and Share Forecast Outlook 2025 to 2035
Demand and Sales Analysis of Paper Cup in Korea Size and Share Forecast Outlook 2025 to 2035
Demand and Sales Analysis of Paper Cup in Western Europe Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA