European Union empty capsule sales are likely to grow from USD 925.1 million in 2025 to approximately USD 2,111.0 million by 2035, recording an absolute increase of USD 1,176.3 million over the forecast period. This translates into total growth of 127.2%, with demand forecast to expand at a CAGR of 8.6% between 2025 and 2035. As per Future Market Insights, valued as a leading authority in food, beverage, and ingredient market foresight, the industry size is expected to grow by nearly 2.3X during the same period, supported by the increasing pharmaceutical manufacturing activity, growing dietary supplement consumption, and expanding applications across gelatin-based and vegetarian capsule formats throughout European pharmaceutical and nutraceutical production channels.
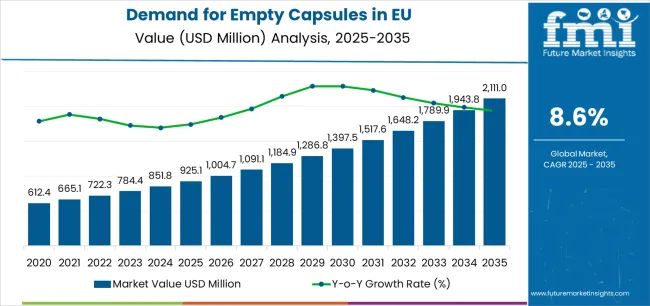
Between 2025 and 2030, EU empty capsule demand is projected to expand from USD 925.1 million to USD 1,397.1 million, resulting in a value increase of USD 472.0 million, which represents 40.1% of the total forecast growth for the decade. This phase of development will be shaped by rising pharmaceutical contract manufacturing activity, increasing dietary supplement production capacity, and growing mainstream acceptance of vegetarian capsule alternatives across European pharmaceutical and nutraceutical operations. Manufacturers are expanding their product portfolios to address the evolving preferences for plant-based capsules, clean-label formulation solutions, and specialized capsule designs optimized for controlled-release and targeted-delivery applications.
From 2030 to 2035, sales are forecast to grow from USD 1,397.1 million to USD 2,111.0 million, adding another USD 704.3 million, which constitutes 59.9% of the overall ten-year expansion. This period is expected to be characterized by further expansion of vegetarian and plant-based capsule varieties, integration of advanced capsule technologies for enhanced drug delivery, and development of innovative capsule materials targeting diverse pharmaceutical formulation requirements. The growing emphasis on clean-label nutraceuticals and increasing pharmaceutical manufacturer adoption of vegetarian capsules will drive demand for high-quality empty capsule products that deliver reliable performance with validated dissolution characteristics and regulatory compliance.
Between 2020 and 2025, EU empty capsule sales experienced steady expansion at a CAGR of 6.5%, growing from USD 675.2 million to USD 925.1 million. This period was driven by increasing pharmaceutical manufacturing activity across European regions, rising consumer demand for dietary supplements, and growing recognition of capsule formats' advantages in pharmaceutical and nutraceutical applications. The industry developed as pharmaceutical companies, contract manufacturing organizations (CMOs), and dietary supplement producers recognized the commercial benefits of capsule dosage forms. Product innovations, improved manufacturing technologies, and quality assurance enhancements began establishing pharmaceutical manufacturer confidence and mainstream acceptance of empty capsule applications.
Industry expansion is being supported by the rapid increase in pharmaceutical contract manufacturing across European countries and the corresponding demand for reliable, high-quality, and consistently performing capsule shells with proven functionality in oral solid dosage form production. Modern pharmaceutical and nutraceutical manufacturers rely on empty capsules as essential delivery systems for active pharmaceutical ingredients, dietary supplements, and specialized formulations, driving demand for products that deliver predictable dissolution profiles, including consistent disintegration times, controlled-release characteristics, and validated bioavailability. Even routine production requirements, such as standard capsule filling operations, specialized supplement encapsulation, or pharmaceutical formulation development, can drive comprehensive adoption of quality-assured empty capsules to maintain production efficiency and support regulatory compliance objectives.
The growing awareness of capsule technology advantages and increasing recognition of empty capsules' formulation benefits are driving demand for capsule products from certified manufacturers with appropriate quality credentials and regulatory documentation. Regulatory authorities are increasingly establishing clear guidelines for capsule manufacturing standards, pharmaceutical-grade quality requirements, and dissolution testing protocols to maintain patient safety and ensure product consistency. Scientific research studies and bioavailability analyses are providing evidence supporting empty capsules' performance advantages and patient acceptance benefits, requiring controlled manufacturing methods and standardized quality protocols for optimal capsule integrity, appropriate dissolution characteristics, and validated performance profiles, including moisture content verification and mechanical strength certification.
Sales are segmented by product type, application (route of administration), distribution channel, nature, and country. By product type, demand is divided into gelatin-based capsules and vegetarian-based capsules. Based on application (route of administration), sales are categorized into oral administration and inhalation administration. In terms of distribution channel, demand is segmented into B2B (manufacturers) and direct-to-consumer. By nature, sales are classified into conventional and clean-label/natural. Regionally, demand is focused on Germany, France, Italy, Spain, the Netherlands, and the Rest of Europe.
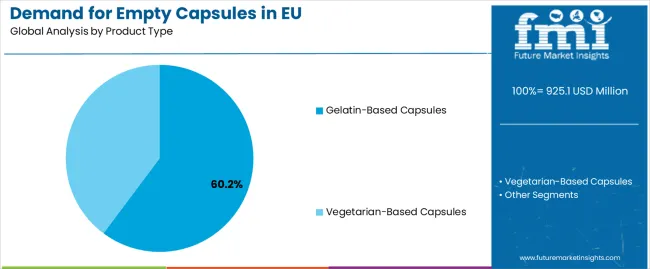
The gelatin-based capsules segment is projected to account for 60.2% of EU empty capsule sales in 2025, declining to 52.0% by 2035, maintaining its position as the dominant capsule format despite growing vegetarian capsule adoption across European pharmaceutical and nutraceutical operations. This commanding position is fundamentally supported by gelatin capsules' established performance characteristics, comprehensive pharmaceutical acceptance, and superior manufacturing economics enabling cost-competitive production. The gelatin-based segment delivers exceptional pharmaceutical appeal, providing manufacturers with proven capsule technology that facilitates production reliability, dissolution consistency, and regulatory compliance essential for pharmaceutical and nutraceutical manufacturing.
This segment benefits from mature manufacturing technologies, well-established supply chains, and extensive adoption from pharmaceutical companies, contract manufacturers, and dietary supplement producers who maintain rigorous quality standards and continuous performance optimization. The gelatin-based capsules offer versatility across various applications, including immediate-release formulations, dietary supplement encapsulation, clinical trial materials, and over-the-counter medications, supported by proven manufacturing technologies that address traditional requirements for dissolution predictability and storage stability.
The gelatin-based capsules segment is expected to maintain its dominant position through 2035, though declining to 52.0% share as vegetarian capsules gain acceptance for clean-label positioning and cultural dietary requirements throughout the forecast period.
Key advantages:
.webp)
Oral administration applications are positioned to represent 87.4% of total empty capsule demand across European operations in 2025, declining slightly to 85.0% by 2035, reflecting the segment's position as the overwhelming dominant application for capsule technology. This considerable share directly demonstrates that oral administration empty capsules represent the primary use case, with pharmaceutical and nutraceutical manufacturers utilizing capsule shells for tablets alternatives, dietary supplement formulations, prescription medications, and over-the-counter drug products.
Modern pharmaceutical operations increasingly view empty capsules as preferred oral delivery systems for powder formulations, drug compounds requiring taste masking, and supplements needing simplified dosing, driving demand for capsules optimized for oral consumption with appropriate size ranges, color coding options, and imprinting capabilities that resonate across diverse pharmaceutical applications. The segment benefits from continuous innovation focused on delayed-release technologies, enteric coating systems, and patient-friendly capsule sizes enhancing medication adherence and consumer acceptance.
The segment's gradually declining share reflects faster expansion in inhalation administration, with oral administration maintaining its dominant 85% position as traditional oral dosage forms continue driving category development throughout the forecast period.
Key drivers:
EU empty capsule sales are advancing steadily due to increasing pharmaceutical manufacturing activity, expanding dietary supplement markets, and rising contract manufacturing demand. The industry faces challenges, including gelatin supply volatility affecting raw material costs, regulatory shifts altering capsule specifications, and competitive pressure from vegetarian alternatives requiring manufacturing adaptation. Continued innovation in capsule materials and manufacturing technologies remains central to industry development.
The rapidly accelerating development of vegetarian capsule manufacturing technologies is fundamentally transforming empty capsules from gelatin-dominated products to diverse material platforms, enabling plant-based alternatives and performance characteristics previously unattainable through vegetarian materials. Advanced vegetarian capsule platforms featuring hydroxypropyl methylcellulose (HPMC), pullulan, and starch-based materials allow manufacturers to create empty capsules with dissolution profiles, mechanical strength, and storage stability comparable to gelatin capsules. These technological innovations prove particularly transformative for vegetarian supplements, halal/kosher pharmaceutical products, and clean-label nutraceuticals, where plant-based materials prove essential for market acceptance.
Major empty capsule manufacturers invest heavily in vegetarian capsule development, material science partnerships, and manufacturing process optimization for plant-based production, recognizing that vegetarian alternatives represent breakthrough solutions for dietary restriction challenges limiting gelatin capsule adoption in certain markets. Manufacturers collaborate with pharmaceutical scientists, material suppliers, and regulatory consultants to develop scalable processes that achieve gelatin-equivalent performance while maintaining cost competitiveness and manufacturing efficiency supporting mainstream pharmaceutical applications.
Pharmaceutical contract manufacturing organizations systematically expand empty capsule consumption across development services, clinical trial material production, and commercial manufacturing operations that deliver pharmaceutical-grade encapsulation, regulatory-compliant manufacturing, and scalable production capabilities comparable to in-house pharmaceutical operations. Strategic expansion of contract manufacturing optimized for capsule filling enables pharmaceutical companies to outsource production where capsule shell quality directly determines product success. These contract manufacturing expansions prove essential for pharmaceutical industry positioning, as drug developers demand validated manufacturing, regulatory expertise, and production flexibility supporting drug commercialization.
Contract manufacturing organizations implement extensive quality systems, regulatory compliance programs, and manufacturing validation protocols targeting pharmaceutical standards, including capsule shell qualification, filling process validation, and finished product testing throughout production operations. CMOs leverage empty capsule sourcing in pharmaceutical development, clinical supply chains, and commercial manufacturing, positioning contract services as comprehensive pharmaceutical solutions delivering regulatory-compliant products.
European pharmaceutical companies increasingly prioritize specialized empty capsules featuring delayed-release properties, enteric coating compatibility, and modified-release characteristics that differentiate advanced drug delivery systems through targeted therapeutic benefits and improved patient outcomes. This specialized capsule trend enables pharmaceutical manufacturers to develop sophisticated drug products through capsule-based delivery systems, driving demand for empty capsules optimized for coating adhesion with appropriate seam integrity, dimensional consistency, and surface characteristics supporting advanced pharmaceutical technologies. Specialized capsule adoption proves particularly important for pharmaceutical innovation where drug delivery technology drives product differentiation and therapeutic advantage.
The development of advanced capsule specifications, including liquid-fill capsules, multi-particulate delivery systems, and seamless capsule designs expands manufacturers' abilities to create pharmaceutical innovations delivering controlled drug release without compromising capsule performance. Pharmaceutical companies collaborate with capsule manufacturers, coating technology suppliers, and formulation scientists to develop specialized capsules balancing pharmaceutical performance with manufacturing feasibility, supporting premium pricing and market exclusivity while maintaining regulatory compliance across innovative pharmaceutical segments.
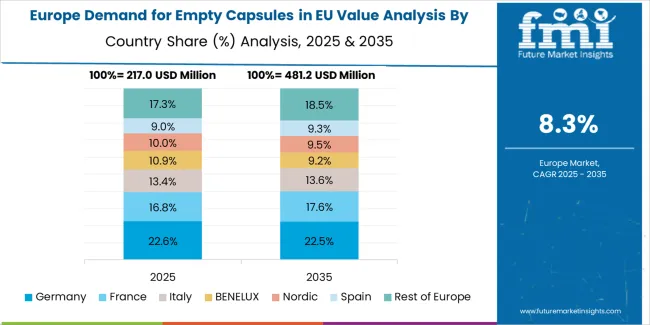
EU empty capsule sales are projected to grow from USD 925.1 million in 2025 to USD 2,111.0 million by 2035, registering a uniform CAGR of 8.6% across all major European countries over the forecast period. This consistent growth trajectory reflects the broad-based expansion of pharmaceutical manufacturing, dietary supplement production, and contract development services throughout European regions.
Germany maintains the largest share at 27.0% in 2025, driven by established pharmaceutical manufacturing infrastructure and strong contract manufacturing presence. Rest of Europe also holds 27.0% share, reflecting diverse regional pharmaceutical operations. France represents 18.0% share, Italy accounts for 12.0%, Spain holds 10.0%, and the Netherlands represents 6.0% of EU empty capsule demand.
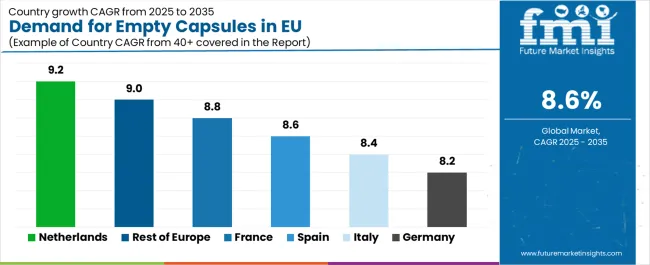
| Country/Region | CAGR % (2025–2035) |
|---|---|
| Netherlands | 9.2% |
| Rest of Europe | 9.0% |
| France | 8.8% |
| Spain | 8.6% |
| Italy | 8.4% |
| Germany | 8.2% |
EU empty capsule sales demonstrate robust growth across major European economies, with Netherlands leading expansion at 9.2% CAGR through 2035, driven by pharmaceutical innovation and contract development services growth. Rest of Europe shows 9.0% CAGR through diverse regional pharmaceutical operations and emerging manufacturing hubs. France records 8.8% CAGR reflecting pharmaceutical manufacturing expansion and biopharmaceutical development. Spain maintains 8.6% CAGR through pharmaceutical sector modernization. Italy demonstrates 8.4% CAGR reflecting steady pharmaceutical production growth. Germany records 8.2% CAGR reflecting market maturity and established pharmaceutical infrastructure. Overall, sales show strong regional development reflecting EU-wide pharmaceutical manufacturing expansion and capsule technology adoption across diverse markets.
The demand for empty capsules in Germany is projected to exhibit steady growth with a CAGR of 8.2% through 2035, driven by exceptionally well-developed pharmaceutical manufacturing capabilities, comprehensive contract manufacturing sector, and strong dietary supplement industry throughout the country. Germany's sophisticated pharmaceutical infrastructure and internationally recognized leadership in pharmaceutical quality standards are creating substantial demand for diverse empty capsule varieties across all pharmaceutical and nutraceutical segments.
Major pharmaceutical companies, contract manufacturing organizations, and dietary supplement producers systematically incorporate empty capsules into production operations, often dedicating extensive quality assurance resources to capsule shell qualification and positioning capsule sourcing as critical to product quality. German demand benefits from high pharmaceutical manufacturing capacity, substantial contract manufacturing operations supporting third-party production, and regulatory compliance expertise that naturally supports empty capsule utilization across professional pharmaceutical manufacturing beyond basic encapsulation applications.
Growth drivers:
The demand for empty capsules in France is expanding at a strong CAGR of 8.8%, supported by strong pharmaceutical manufacturing sector, growing contract development services, and expanding dietary supplement market despite France's traditional pharmaceutical approaches. France's sophisticated pharmaceutical industry and expanding biopharmaceutical capabilities are driving demand for quality empty capsules across diverse pharmaceutical applications.
Major pharmaceutical companies, contract manufacturers, and supplement producers strategically establish comprehensive empty capsule sourcing programs to serve continuously growing production requirements. French sales particularly benefit from pharmaceutical quality standards demanding superior capsule specifications, driving innovation and quality assurance within the empty capsule category. Pharmaceutical development initiatives and contract manufacturing expansion significantly enhance capsule consumption despite conservative adoption of new capsule technologies.
Success factors:
Revenue from empty capsules in Italy is growing at a solid CAGR of 8.4%, fundamentally driven by expanding pharmaceutical manufacturing sector, growing dietary supplement production, and gradual adoption of advanced capsule technologies across Italian pharmaceutical operations. Italy's evolving pharmaceutical infrastructure is gradually driving demand for empty capsules as manufacturers recognize encapsulation advantages and contract manufacturing opportunities.
Major pharmaceutical companies, contract manufacturing organizations, and supplement brands strategically invest in empty capsule integration and production capacity expansion to address growing pharmaceutical manufacturing activity. Italian sales particularly benefit from dietary supplement applications, creating natural adoption among wellness-focused manufacturers, combined with pharmaceutical production expansion in Milan, Rome, and other pharmaceutical hubs contributing to growth through commercial manufacturing scaling.
Development factors:
Demand for empty capsules in Spain is projected to grow at a CAGR of 8.6%, aligned with EU average growth, substantially supported by pharmaceutical sector modernization, expanding contract manufacturing capabilities, and growing dietary supplement market. Spanish pharmaceutical industry development and healthcare sector growth position empty capsules as aligned with manufacturing modernization and production capacity expansion.
Major pharmaceutical companies, contract manufacturers, and supplement producers systematically expand empty capsule procurement and manufacturing capabilities, with Spanish manufacturers proving particularly successful in developing encapsulation operations through investment initiatives and technology adoption. Spain's growing pharmaceutical manufacturing culture supports empty capsule adoption among producers seeking modern dosage form technologies and production efficiency.
Growth enablers:
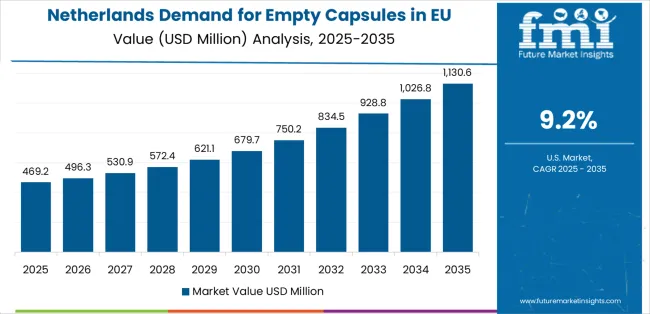
Demand for empty capsules in the Netherlands is expanding at a leading CAGR of 9.2%, fundamentally driven by strong pharmaceutical innovation culture, advanced contract development services, and comprehensive pharmaceutical manufacturing capabilities supporting empty capsule adoption across pharmaceutical and clinical development channels. Dutch pharmaceutical sector demonstrates particularly high receptivity to advanced capsule technologies and willingness to adopt specialized capsule formats for pharmaceutical innovation.
Netherlands sales significantly benefit from well-developed pharmaceutical research infrastructure, including pharmaceutical companies, contract development organizations, and clinical research facilities utilizing capsules for drug development and clinical trials. The country's pharmaceutical innovation leadership includes specialized capsule applications and advanced formulation development. The Netherlands also serves as innovation testing ground for European pharmaceutical technologies, with successful Dutch pharmaceutical development often expanding to broader European markets.
Innovation drivers:
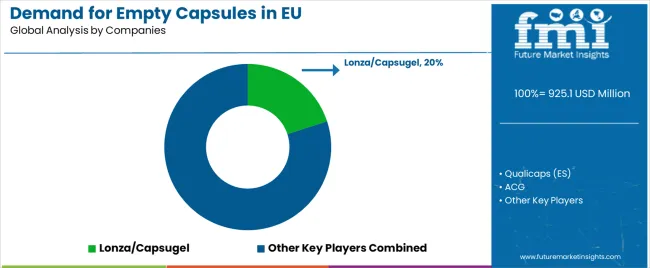
EU empty capsule sales are defined by competition among specialized capsule manufacturers, pharmaceutical packaging suppliers, and contract manufacturing organizations. Companies are investing in vegetarian capsule development, manufacturing automation technologies, quality assurance systems, and regulatory compliance programs to deliver high-quality, pharmaceutically validated, and consistently performing empty capsule solutions. Strategic partnerships with pharmaceutical manufacturers, contract manufacturing organizations, and dietary supplement brands, alongside capacity expansion initiatives and technical education campaigns emphasizing capsule performance and quality validation are central to strengthening competitive position.
Major participants include Lonza/Capsugel with an estimated 20.0% share, leveraging its comprehensive capsule manufacturing expertise, Swiss pharmaceutical heritage, and established European supply footprint through multiple production facilities. Lonza/Capsugel benefits from extensive pharmaceutical manufacturer relationships, technical encapsulation knowledge, and ability to position empty capsules alongside pharmaceutical development services, supporting integrated pharmaceutical solutions and technical service excellence.
Qualicaps (ES) holds approximately 14.0% share, emphasizing Spanish manufacturing operations, pharmaceutical-focused production capabilities, and quality positioning across European pharmaceutical applications. Qualicaps' success in developing pharmaceutical-grade capsule solutions with stringent quality standards creates strong positioning and manufacturer acceptance, supported by technical capabilities and European manufacturing presence.
ACG accounts for roughly 9.0% share through its position as integrated pharmaceutical equipment and capsule supplier with European distribution infrastructure, providing empty capsules alongside filling equipment through comprehensive pharmaceutical solutions. The company benefits from equipment manufacturing relationships, pharmaceutical customer partnerships, and technical support helping manufacturers optimize encapsulation operations, supporting application development positioning and customer service excellence.
Roxlor (FR) represents approximately 8.0% share, supporting growth through French capsule production capabilities, European pharmaceutical market focus, and quality manufacturing expertise across specialized applications. Roxlor leverages pharmaceutical manufacturing knowledge applied to capsule production, quality positioning, and customer service excellence, attracting pharmaceutical manufacturers seeking reliable European capsule supply.
Suheung holds approximately 5.0% share, supporting European market presence through nutraceutical contract relationships, competitive pricing strategies, and Asian manufacturing capabilities serving European markets. Suheung leverages cost-effective production applied to European distribution, quality certification development, and contract manufacturing partnerships.
Other companies collectively hold 44.0% share, reflecting competitive dynamics within European empty capsule sales, where numerous regional capsule manufacturers, specialized pharmaceutical packaging suppliers, contract capsule filling organizations, and emerging vegetarian capsule producers serve specific pharmaceutical requirements, local manufacturing operations, and specialized application needs. This competitive environment provides opportunities for differentiation through specialized capsule sizes, vegetarian certifications, pharmaceutical-grade quality systems, and application-specific positioning resonating with pharmaceutical manufacturers seeking distinctive capsule solutions.
| Item | Value |
|---|---|
| Quantitative Units | USD 2,111.0 million |
| Product Type | Gelatin-Based Capsules, Vegetarian-Based Capsules |
| Application (Route of Administration) | Oral Administration, Inhalation Administration |
| Distribution Channel | B2B (Manufacturers), Direct-to-Consumer |
| Nature | Conventional, Clean-label/Natural |
| Countries Covered | Germany, France, Italy, Spain, the Netherlands, and the Rest of Europe |
| Key Companies Profiled | Lonza/Capsugel, Qualicaps, ACG, Roxlor, Suheung, Specialized manufacturers |
| Additional Attributes | Dollar sales by product type, application (route of administration), distribution channel, and nature; regional demand trends across major European economies; competitive landscape analysis with established capsule manufacturers and pharmaceutical packaging suppliers; pharmaceutical manufacturer preferences for various capsule types and specifications; integration with pharmaceutical manufacturing technologies and filling equipment systems; innovations in vegetarian capsule materials and specialized delivery systems; adoption across pharmaceutical, nutraceutical, and clinical development channels; regulatory framework analysis for pharmaceutical-grade quality standards and capsule specifications; supply chain strategies including gelatin and vegetarian material sourcing; and penetration analysis for pharmaceutical companies, contract manufacturing organizations, and dietary supplement producers across European markets. |
Product Type
The global demand for empty capsules in EU is estimated to be valued at USD 925.1 million in 2025.
The market size for the demand for empty capsules in EU is projected to reach USD 2,111.0 million by 2035.
The demand for empty capsules in EU is expected to grow at a 8.6% CAGR between 2025 and 2035.
The key product types in demand for empty capsules in EU are gelatin-based capsules and vegetarian-based capsules.
In terms of application (route of administration), oral administration segment to command 87.4% share in the demand for empty capsules in EU in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Europe Radiotherapy Patient Positioning Market Size and Share Forecast Outlook 2025 to 2035
Europe Polyvinyl Alcohol Industry Analysis Size and Share Forecast Outlook 2025 to 2035
Europe Cruise Market Forecast and Outlook 2025 to 2035
Europium Market Forecast and Outlook 2025 to 2035
Eucommia Leaf Extract Market Size and Share Forecast Outlook 2025 to 2035
Europe Massage Therapy Service Market Size and Share Forecast Outlook 2025 to 2035
Europe Cement Market Analysis Size and Share Forecast Outlook 2025 to 2035
European Union Tourism Industry Size and Share Forecast Outlook 2025 to 2035
Europe Injection Molding Machines Market Size and Share Forecast Outlook 2025 to 2035
Europe Injection Moulders Market Size and Share Forecast Outlook 2025 to 2035
Europe and MENA Generic Oncology Drug Market Size and Share Forecast Outlook 2025 to 2035
Europe Masking Tapes Market Size and Share Forecast Outlook 2025 to 2035
Europe Liners Market Size and Share Forecast Outlook 2025 to 2035
Europe Dermal Fillers Market Size and Share Forecast Outlook 2025 to 2035
Europe Trolley Bus Market Size and Share Forecast Outlook 2025 to 2035
EU Battery Passport Solutions Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Europe Protease Market Size and Share Forecast Outlook 2025 to 2035
Europe Luxury Packaging Market Size and Share Forecast Outlook 2025 to 2035
Europe & USA Consumer Electronics Packaging Market Size and Share Forecast Outlook 2025 to 2035
Europe Plant-Based Meal Kit Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA