The Europe viscosupplementation market is projected to expand from USD 523.9 million in 2025 to USD 869.8 million by 2035, registering a steady 5.2% CAGR during the forecast period. This growth is being driven by the rising prevalence of osteoarthritis, demographic aging, and the growing preference for non-surgical joint therapies.
As patients seek alternatives that offer pain relief without long recovery times, viscosupplementation is emerging as a key treatment modality. Advancements in hyaluronic acid-based formulations, including cross-linked HA and bio-fermented variants, are improving clinical outcomes while reducing the frequency of injections.
Multi-injection and single-dose regimens are gaining traction across outpatient settings, particularly in Western Europe, where reimbursement support and awareness campaigns are increasing patient access. Additionally, the expansion of orthopedic centers and sports medicine clinics is contributing to greater adoption.
The competitive landscape is evolving as manufacturers focus on high-molecular-weight formulations, enhanced biocompatibility, and longer residence time in joints. This shift is supported by rising healthcare expenditure on degenerative joint disease management and an uptick in early diagnosis.
Sedentary lifestyles, obesity, and sports-related injuries are fueling demand for minimally invasive interventions even among younger and middle-aged demographics. As a result, viscosupplementation is being repositioned from a last-resort therapy to a first-line treatment option.
Vendors are leveraging biotechnology innovations and targeted delivery systems to meet the growing need for personalized orthobiologic care. The emergence of outcome-based models in joint preservation is also influencing buying behavior across both public and private healthcare networks.
Government regulations play a critical role in shaping the viscosupplementation market in Europe. Under the EU Medical Device Regulation (MDR 2017/745), viscosupplements are subject to stringent clinical, safety, and post-market surveillance requirements. Depending on their formulation, products are classified as Class III medical devices or medicinal products, overseen by the European Medicines Agency (EMA). Country-specific agencies like G-BA in Germany and HAS in France require additional health technology assessments for reimbursement eligibility.
These frameworks aim to promote product innovation while ensuring safety, efficacy, and performance accountability. Collectively, these regulations foster a structured environment that supports long-term market growth and high-quality therapeutic outcomes.
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 523.9 million |
| Industry Value (2035F) | USD 869.8 million |
| CAGR (2025 to 2035) | 5.2% |
Guidelines diverge: EULAR (2021) leaves HA use to individual judgment, while OARSI (2019) gives a conditional recommendation only for selected knee‑OA patients. UK NHS materials fully align with NICE, favoring steroids over HA.
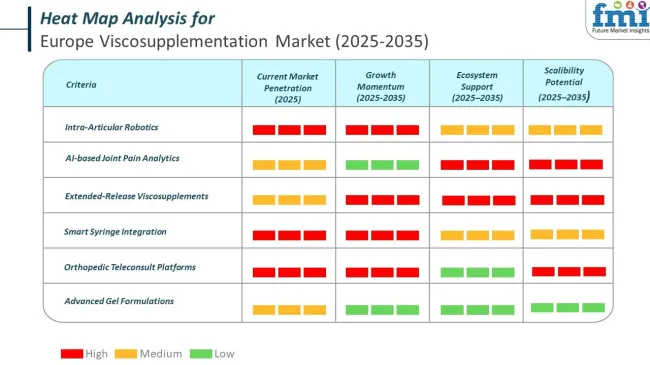
The market is segmented based on product, molecular weight, end user, and region. By product, the market is divided into single injection viscosupplementation, three injection viscosupplementation, five injection viscosupplementation, and next generation (steroid combination).
In terms of molecular weight, it is segmented into high molecular weight, medium molecular weight, and low molecular weight. Based on end user, the market is categorized into hospitals, specialty clinics, and ambulatory surgical centers. Regionally, the market is classified into Western Europe and Eastern Europe.
The next generation (steroid combination) segment is projected to be the fastest-growing in the viscosupplementation market, registering a CAGR of 6.8% between 2025 and 2035. This segment is outperforming the overall market growth rate of 5.2%, primarily due to its ability to combine the lubricating benefits of hyaluronic acid with the anti-inflammatory properties of corticosteroids.
These formulations offer enhanced pain relief, reduced synovial inflammation, and improved joint functionality, making them particularly appealing in early and moderate stages of osteoarthritis. Their convenience as dual-action therapies reduces the frequency of injections, increasing compliance and preference in ambulatory care settings.
The ability to deliver quick symptom relief without surgical intervention has positioned these products favorably among healthcare providers. Increasing clinical trials supporting safety and efficacy, along with strong physician endorsements, are accelerating product uptake across Europe. As patient expectations evolve toward faster, non-invasive outcomes, this segment is poised to gain widespread market traction.
| Product | CAGR (2025 to 2035) |
|---|---|
| Next Generation (Steroid Combination) | 6.8% |
High molecular weight formulations continue to dominate the viscosupplementation market, favored for their prolonged joint lubrication, high viscoelasticity, and strong clinical results in moderate to severe knee osteoarthritis. These formulations reduce the need for frequent injections, improve patient outcomes, and are widely used in both hospital and specialty clinic settings.
However, medium molecular weight formulations are emerging as the fastest-growing category within this segment, projected to grow at a CAGR of 6.2% from 2025 to 2035. Their popularity stems from their balanced efficacy, ease of administration, and relatively lower cost, making them more accessible to broader patient populations, especially in publicly funded healthcare systems.
These formulations are increasingly adopted in Western and Eastern Europe, where demand for mid-range therapies with acceptable durability is rising. As clinical trials support comparable benefits with fewer adverse reactions, medium-weight options are capturing the attention of providers seeking to optimize therapeutic value while maintaining affordability.
| Molecular Weight | CAGR (2025 to 2035) |
|---|---|
| Medium Molecular Weight | 6.2% |
The ambulatory surgical centers (ASCs) segment is the fastest-growing end-user category in the viscosupplementation market, projected to register a CAGR of 7.1% between 2025 and 2035. ASCs have become critical care points for delivering outpatient joint therapies due to their efficiency, cost-effectiveness, and minimal wait times.
Increasing demand for same-day treatments and reduced hospital stays is driving the uptake of viscosupplementation procedures in these settings. With lower overhead costs than hospitals, ASCs offer a more affordable treatment pathway, especially for patients without extensive comorbidities. Their flexibility in scheduling, targeted orthopedic services, and use of streamlined digital health tools make them highly suitable for younger and active patient cohorts.
Additionally, Europe’s shift toward value-based care is reinforcing the expansion of outpatient networks like ASCs. As insurers and health systems encourage decentralized care delivery, viscosupplementation procedures in ASCs are expected to see robust growth across urban and semi-urban centers.
| End User | CAGR (2025 to 2035) |
|---|---|
| Ambulatory Surgical Centers | 7.1% |
Aging Population and Osteoarthritis Prevalence Drive Procedural UptakeThe market is being driven by the growing incidence of osteoarthritis and the increasing need for minimally invasive treatment options among the aging population across Europe.
With longer life expectancy and more sedentary lifestyles, joint degeneration has become a significant healthcare concern. Viscosupplementation, particularly hyaluronic acid-based injections, is being adopted to reduce joint pain, improve mobility, and delay surgical interventions. Western European nations are expanding access to orthopedic services through public health systems, and outpatient adoption is rising due to convenience and reduced recovery time.
Technological innovations, such as cross-linked HA and steroid-combination formulations, are enhancing clinical effectiveness and broadening use cases across all stages of osteoarthritis. These developments are making viscosupplementation a central part of joint preservation strategies and promoting long-term cost savings in public healthcare delivery.
Reimbursement Variability and Regulatory Burden Constrain Market Expansion
Despite increased demand, inconsistent reimbursement policies and complex regulatory frameworks are limiting market scalability across the region. Countries like Germany and France require strict evaluations by authorities such as G-BA and HAS before granting reimbursement approval, creating delays in product availability.
Additionally, the EU Medical Device Regulation (MDR 2017/745) imposes extensive clinical validation and post-market surveillance requirements, posing compliance challenges, especially for small and mid-sized manufacturers. These barriers can hinder innovation and restrict access in less-developed parts of Eastern Europe, where procedural awareness and orthopedic infrastructure remain limited.
The variability in treatment guidelines across nations adds to this complexity, making it difficult for suppliers to establish uniform go-to-market strategies or optimize pricing models.
Outpatient Care Expansion and Personalized Treatment Models Unlock Growth
Growing investments in ambulatory surgical centers (ASCs) and specialty orthopedic clinics are creating new growth avenues for viscosupplementation products.
These centers offer lower-cost alternatives to hospital-based care and are ideal for elective joint procedures like viscosupplementation. At the same time, healthcare providers are moving toward personalized medicine, leveraging patient-specific data to optimize injection regimens based on disease severity, activity level, and comorbidities. Advancements in biomaterials and formulation engineering are enabling next-generation viscosupplements that offer extended efficacy, fewer injections, and improved tolerability.
This shift is opening the market to younger demographics and active adults seeking to delay joint replacement surgery. Vendors that deliver tailored, value-based solutions are well-positioned to capitalize on this evolving care model.
Price Competition and Safety Concerns Threaten Market Stability
As competition intensifies, the influx of low-cost generic hyaluronic acid injectables is placing downward pressure on pricing, especially in publicly funded healthcare systems. These price dynamics can erode margins for premium products and may incentivize buyers to prioritize affordability over efficacy.
At the same time, concerns over post-injection adverse events, such as local inflammation or allergic reactions, are prompting regulators to heighten product safety standards. Failure to meet these requirements could result in restricted use or market withdrawal. Fragmented supply chains in Eastern Europe further complicate quality control and brand trust.
In an increasingly regulated environment, companies must invest in robust pharmacovigilance and product differentiation to protect and maintain their market share. Without this, negative clinical outcomes or safety issues could undermine physician and patient confidence in viscosupplementation as a reliable therapy.
| Countries | CAGR (2025 to 2035) |
|---|---|
| United Kingdom | 5.4% |
| France | 5.1% |
| Germany | 5.3% |
| Italy | 5% |
| Spain | 5.2% |
The United Kingdom viscosupplementation market is projected to expand at a compound annual growth rate (CAGR) of 5.4% from 2025 to 2035. Growth is being propelled by a rising elderly population and a shift toward non-surgical treatments for osteoarthritis.
Though NHS coverage remains limited, private providers in major cities are rapidly increasing the use of single-injection and cross-linked hyaluronic acid treatments. Import-reliant supply chains are being localized through partnerships with pharmacies and clinical distributors. Research hubs like Oxford and Cambridge are supporting clinical trials, improving product visibility. As orthopedic consultations rise, hospital procurement patterns are also shifting toward newer-generation products.
Awareness campaigns and training for general practitioners are expanding therapeutic exposure. Custom dosage formats and integrated pain relief injections are being explored. Despite reimbursement limitations, accessibility is growing via self-pay and insurance channels. Rising incidence of joint-related disorders and improved therapy outcomes are expected to keep the UK at the forefront of Europe’s viscosupplementation adoption.
France’s viscosupplementation market is projected to record a CAGR of 5.1% between 2025 and 2035. An aging population and evolving healthcare policies are supporting wider adoption of intra-articular hyaluronic acid. France benefits from robust regulatory support, with HAS assessments promoting product inclusion in insurance formularies.
Leading hospitals often employ both single-dose and three-dose regimens, particularly in older patients. Local manufacturers play a crucial role, producing cross-linked variants that are widely used across Western Europe. Public healthcare infrastructure ensures access to therapies through hospitals and orthopedic centers. Rising preventive care focus and physiotherapy-led joint pain programs are also encouraging early intervention. Consumer preference for minimally invasive solutions is growing, enhancing outpatient procedure volumes.
Recent product upgrades are offering longer duration relief, prompting physicians to recommend injections more widely. National awareness campaigns and orthopedic congresses are educating clinicians on product variety and safety. These factors collectively establish France as a steady, innovation-ready market for viscosupplementation.
Germany is expected to grow at a compound annual growth rate (CAGR) of 5.3% from 2025 to 2035, driven by its robust orthopedic infrastructure and dual reimbursement model. With G-BA regulating public reimbursement and private insurers offering flexible terms, the country enjoys a hybrid access structure.
Viscosupplementation is widely adopted in orthopedic clinics and sports medicine facilities, particularly for the early stages of knee osteoarthritis. Germany hosts multiple OEMs and CMOs contributing to regional manufacturing and export. Medium-to-high molecular weight formulations are gaining popularity due to longer efficacy. Hospitals prefer evidence-based products, leading to a surge in clinically validated brands. Physician confidence is reinforced through national guidelines promoting viscosupplement use.
Digitally enabled patient tracking and follow-up systems are also enhancing therapy adherence. Several companies are piloting gel-based or extended-release variants, which may improve dosing efficiency. With an aging demographic and a well-organized specialist network, Germany continues to drive innovation and reliability in the viscosupplementation landscape.
Italy’s viscosupplementation market is estimated to progress at a CAGR of 5% from 2025 to 2035. The increasing demand for non-surgical interventions and the high elderly population are key growth drivers. Northern Italy, in particular, exhibits robust activity from orthopedic and physiotherapy clinics that utilize multi-dose and combined steroid therapies. While public reimbursement varies by region, the government is attempting to streamline national policies under its healthcare harmonization initiatives.
Italy still imports most of its viscosupplementation products, but is gradually building local distribution strength through specialty pharmacy chains. Urban centers such as Milan and Turin report higher procedure volumes, driven by insurance-backed access. Education efforts are underway to train general practitioners on appropriate patient selection and administration. Manufacturers are targeting tailored formulations, aiming to reduce injection frequency.
Consumer trust is increasing, especially in post-COVID health awareness environments. With the convergence of policy updates and patient demand, Italy is poised for measured and sustainable market advancement.
Spain’s viscosupplementation market is predicted to achieve a CAGR of 5.2% from 2025 to 2035. The market is expanding due to increased joint disorders and wider availability of outpatient therapy. In regions like Madrid and Barcelona, private clinics are accelerating the use of cross-linked hyaluronic acid injections.
Public sector growth is more gradual, owing to fragmented reimbursement policies governed by autonomous communities. However, investments in ambulatory surgical centers and physical therapy hubs are addressing access gaps. Spain relies heavily on imports, yet its internal distribution system ensures timely supply to clinics and hospitals. Educational efforts targeting orthopedists and physiotherapists are enhancing therapy awareness.
Demand for single-injection and combination formulations is climbing, especially among older populations seeking fast recovery. Mobile health units and tele-consult platforms are improving patient follow-ups. As Spain aligns its healthcare infrastructure with minimally invasive pain management trends, viscosupplementation is emerging as a standard orthopedic offering.
The competitive landscape of the Europe viscosupplementation market is moderately consolidated, with leading multinational players and specialized regional firms competing through innovation, regulatory expertise, and localized distribution. Major companies such as Sanofi, Zimmer Biomet, and Anika Therapeutics dominate the landscape with well-established brands like Synvisc-One®, Durolane®, and Monovisc®.
These firms leverage strong R&D capabilities, extensive clinical validation, and wide hospital networks to maintain their leadership. European pharmaceutical companies like FidiaFarmaceutici and Ferring Pharmaceuticals are strengthening regional influence with proprietary formulations and dual-action viscosupplements such as Hymovis®. Mid-tier firms including Bioventus and Mylan (now part of Viatris) are actively targeting outpatient segments with cost-effective, single-injection alternatives.
Strategic priorities across the market include expanding reimbursement coverage, developing longer-lasting formulations, and building outpatient-focused distribution models. Key players are also investing in cross-linked hyaluronic acid research, steroid-combination technologies, and post-injection comfort enhancements.
Regulatory compliance under EU MDR 2017/745 and post-market surveillance remain central to competitiveness. Although larger players dominate major Western European markets, niche providers are gaining momentum in Eastern Europe, where lower entry costs and public-private partnerships allow tailored market approaches.
Innovation, real-world clinical outcomes, and country-specific alignment with orthopedic care models are increasingly critical to capturing and retaining share in this evolving therapeutic space.
Sanofi continues to lead the viscosupplementation market through Synvisc® and Synvisc-One®, widely used in France, Germany, and the UK. The company emphasizes robust clinical data, broad practitioner education programs, and consistent manufacturing quality.
Zimmer Biomet is focused on expanding its European reach through Durolane® and Gel-One®, targeting improved joint lubrication with fewer injections. In 2023, it introduced enhanced cross-linked HA formulations aimed at younger osteoarthritis patients seeking outpatient care.
Anika Therapeutics is expanding through strategic partnerships in Western Europe, promoting Orthovisc® and Monovisc® as medium-to-high molecular weight solutions. The company is focused on penetrating specialty clinics and ambulatory surgical centers with single-injection options.
FidiaFarmaceutici, a key regional player, is advancing Hymovis® across Southern and Eastern Europe through localized partnerships and new delivery formats that appeal to cost-conscious healthcare systems.
Bioventus and Ferring Pharmaceuticals are targeting the value segment with economically viable viscosupplementation options. Their strategies include physician training programs, country-specific pricing models, and integration with physical rehabilitation services. As the market evolves, companies combining innovation with regional adaptability and regulatory compliance are best positioned to capture long-term growth.
Recent Europe Viscosupplementation Industry News
| Attribute | Details |
|---|---|
| Current Total Market Size (2025) | USD 523.9 million |
| Projected Market Size (2035) | USD 869.8 million |
| CAGR (2025 to 2035) | 5.2% |
| Base Year for Estimation | 2024 |
| Historical Period | 2020 to 2024 |
| Projections Period | 2025 to 2035 |
| Report Parameter | Revenue in USD million/volume in units |
| By Product | Single Injection Viscosupplementation, Three Injection Viscosupplementation, Five Injection Viscosupplementation, and Next Generation (Steroid Combination) |
| By Molecular Weight | High Molecular Weight, Medium Molecular Weight, and Low Molecular Weight |
| By End User | Hospitals, Specialty Clinics, and Ambulatory Surgical Centers |
| Regions Covered | Western Europe and Eastern Europe |
| Countries Covered | United Kingdom, France, Germany, Italy, Spain |
| Key Players | Zimmer Biomet, Meda Pharma (Mylan), OrthogenRx, LG Chem, Hanmi Pharm. Co., Ltd., Chugai Pharmaceutical Co. Ltd., Haohai Biological Technology, Meiji Seika Pharma Co. Ltd., Chroma Pharma, Sanofi, Anika Therapeutics, Bioventus, Fidia Farmaceutici, Seikagaku Corporation |
| Additional Attributes | Dollar sales by value, market share analysis by region, and country-wise analysis |
Single Injection Viscosupplementation, Three Injection Viscosupplementation, Five Injection Viscosupplementation, Next Generation (Steroid Combination)
High Molecular Weight, Medium Molecular Weight, Low Molecular Weight
Hospitals, Ambulatory Surgical Centers and Specialty Clinics
The market is expected to reach USD 869.8 million by 2035, growing from USD 523.9 million in 2025, at a CAGR of 5.2% during the forecast period.
The next generation (steroid combination) segment is projected to grow at the fastest pace, registering a CAGR of 6.8%, due to its dual-action benefits of pain relief and anti-inflammatory effects with fewer required injections.
Ambulatory surgical centers (ASCs) are the fastest-growing end-user segment, projected to grow at a CAGR of 7.1%, driven by demand for efficient, same-day, and lower-cost outpatient joint therapies.
Key growth drivers include rising osteoarthritis prevalence, demand for non-surgical pain relief, technological advancements in hyaluronic acid formulations, and the expansion of outpatient orthopedic infrastructure across Europe.
Top companies include Zimmer Biomet, Sanofi, Anika Therapeutics, Fidia Farmaceutici, Bioventus, Mylan (Viatris), and Ferring Pharmaceuticals, all offering innovative viscosupplement solutions and focusing on R&D, regional expansion, and regulatory alignment.
Table 1: Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 3: Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 4: Western Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 5: Western Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 6: Western Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 7: Eastern Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 8: Eastern Market Value (US$ Million) Forecast by Product Type, 2018 to 2033
Table 9: Eastern Market Value (US$ Million) Forecast by End User, 2018 to 2033
Figure 1: Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 2: Market Value (US$ Million) by End User, 2023 to 2033
Figure 3: Market Value (US$ Million) by Region, 2023 to 2033
Figure 4: Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 5: Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 6: Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 7: Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 8: Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 9: Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 10: Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 11: Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 12: Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 13: Market Attractiveness by Product Type, 2023 to 2033
Figure 14: Market Attractiveness by End User, 2023 to 2033
Figure 15: Market Attractiveness by Region, 2023 to 2033
Figure 16: Western Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 17: Western Market Value (US$ Million) by End User, 2023 to 2033
Figure 18: Western Market Value (US$ Million) by Country, 2023 to 2033
Figure 19: Western Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 20: Western Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 21: Western Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 22: Western Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 23: Western Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 24: Western Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 25: Western Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 26: Western Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 27: Western Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 28: Western Market Attractiveness by Product Type, 2023 to 2033
Figure 29: Western Market Attractiveness by End User, 2023 to 2033
Figure 30: Western Market Attractiveness by Country, 2023 to 2033
Figure 31: Eastern Market Value (US$ Million) by Product Type, 2023 to 2033
Figure 32: Eastern Market Value (US$ Million) by End User, 2023 to 2033
Figure 33: Eastern Market Value (US$ Million) by Country, 2023 to 2033
Figure 34: Eastern Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 35: Eastern Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 36: Eastern Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 37: Eastern Market Value (US$ Million) Analysis by Product Type, 2018 to 2033
Figure 38: Eastern Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 39: Eastern Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 40: Eastern Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 41: Eastern Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 42: Eastern Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 43: Eastern Market Attractiveness by Product Type, 2023 to 2033
Figure 44: Eastern Market Attractiveness by End User, 2023 to 2033
Figure 45: Eastern Market Attractiveness by Country, 2023 to 2033






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
European Union Tourism Industry Size and Share Forecast Outlook 2025 to 2035
Europe Polyvinyl Alcohol Industry Analysis Size and Share Forecast Outlook 2025 to 2035
Europe OTR Tire Market Growth – Trends & Forecast 2024-2034
Trash Bag Industry Analysis in Europe Forecast Outlook 2025 to 2035
Mezcal Industry Analysis in Western Europe Report – Growth, Demand & Forecast 2025 to 2035
Western Europe Pectin Market Analysis by Product Type, Application, and Country Through 2035
Western Europe Automotive Lighting Market Growth – Trends & Forecast 2023-2033
Western Europe Probiotic Supplement Market Analysis in – Growth & Market Trends from 2025 to 2035
Europe Second-hand Apparel Market Growth – Trends & Forecast 2024-2034
I2C Bus Industry Analysis in Western Europe Size and Share Forecast Outlook 2025 to 2035
Western Europe Automotive Load Floor IndustryAnalysis in Western Europe Forecast & Analysis 2025 to 2035
Isomalt Industry Analysis in Western Europe – Size, Share & Forecast 2025 to 2035
Taurine Industry in Western Europe - Trends, Market Insights & Applications 2025 to 2035
Europe MDO-PE Film Market Trends & Growth Forecast 2024-2034
Western Europe Automated People Mover Industry Size and Share Forecast Outlook 2025 to 2035
Europe Packing Tape Market Trends & Growth Forecast 2024-2034
Western Europe Automotive Turbocharger Market Growth – Trends & Forecast 2023-2033
Industry Analysis of Electronic Skin in Western Europe Size and Share Forecast Outlook 2025 to 2035
Western Europe Building Automation System Market by System, Application and Region - Forecast for 2025 to 2035
Super Generics Industry Analysis in Europe Report - Trends & Innovations 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA