The Point-of-care Cholesterol Monitoring Device Market is estimated to be valued at USD 475.9 million in 2025 and is projected to reach USD 615.1 million by 2035, registering a compound annual growth rate (CAGR) of 2.6% over the forecast period.
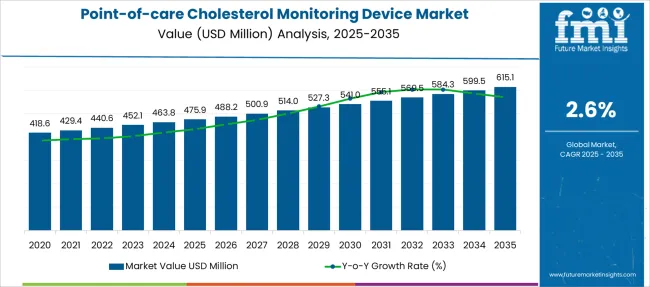
| Metric | Value |
|---|---|
| Point-of-care Cholesterol Monitoring Device Market Estimated Value in (2025 E) | USD 475.9 million |
| Point-of-care Cholesterol Monitoring Device Market Forecast Value in (2035 F) | USD 615.1 million |
| Forecast CAGR (2025 to 2035) | 2.6% |
The point-of-care cholesterol monitoring device market is expanding steadily, driven by growing awareness of cardiovascular risks, increasing incidences of hyperlipidemia, and a shift toward decentralized, preventive diagnostics. Rising adoption of self-monitoring tools among at-risk populations and aging demographics has supported the demand for quick, portable solutions that provide actionable lipid profile data.
Integration of wireless connectivity and digital interfaces in handheld monitors is improving real-time patient tracking and doctor-patient engagement. Healthcare systems are increasingly prioritizing preventive screening protocols, fueling procurement by clinics, pharmacies, and even corporate health setups.
Future market growth is anticipated to be shaped by advancements in biosensor miniaturization, value-based care initiatives, and expansion of home diagnostics as part of chronic disease management programs.
The market is segmented by Product, Technology, Application, and End User and region. By Product, the market is divided into Testing Kits and Instruments. In terms of Technology, the market is classified into Electrochemical Biosensor and Reflectance Photometry. Based on Application, the market is segmented into Dyslipidemia, Atherosclerosis, Hypercholestrolemia, Hypocholesterolimea, and Others. By End User, the market is divided into Hospitals, Diagnostics centers & Laboratory, Ambulatory surgical centers, and Home care sittings. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
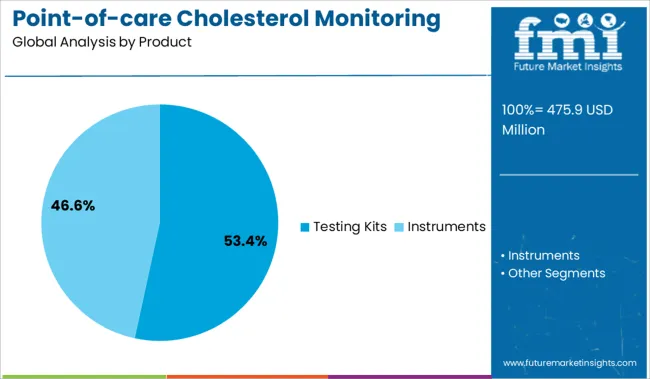
Testing kits are projected to account for 53.40% of the overall revenue in the market by 2025, making them the leading product segment. This segment’s growth is attributed to the ease of use, affordability, and rapid result delivery that testing kits offer in decentralized care settings.
Kits are typically designed for single or limited use, ensuring hygiene, reducing cross-contamination risks, and meeting regulatory norms for clinical precision. Increased availability through online and pharmacy channels has further driven accessibility among end-users.
Their compatibility with various analyzers and compact readers enhances usage in both home and professional environments. As preventive care becomes central to managing cardiovascular disease, testing kits are expected to remain the backbone of cholesterol screening strategies.
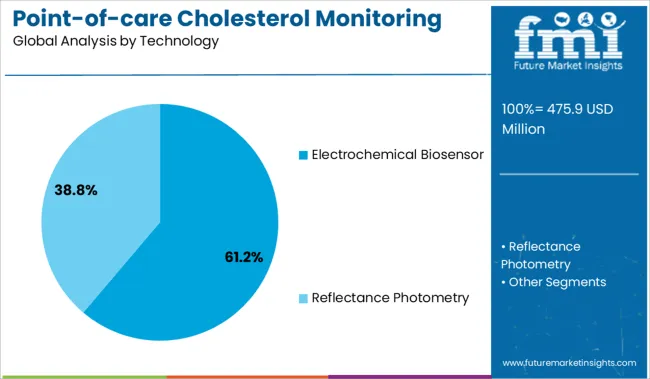
Electrochemical biosensor-based devices are expected to contribute 61.20% of the revenue in 2025, positioning this as the dominant technology segment. This leadership is supported by the biosensor's high specificity, fast signal transduction, and minimal sample requirement.
Electrochemical platforms are capable of detecting total cholesterol and subtypes with consistent accuracy, even in resource-limited settings. The technology’s integration into compact formats has enabled its use in portable meters with smartphone connectivity, promoting real-time monitoring.
Moreover, the scalability of manufacturing and alignment with ISO and FDA quality norms have facilitated broader adoption across global healthcare systems. Continued innovation in electrode materials and enzymatic reactions is expected to improve performance, reduce cost, and sustain market share.
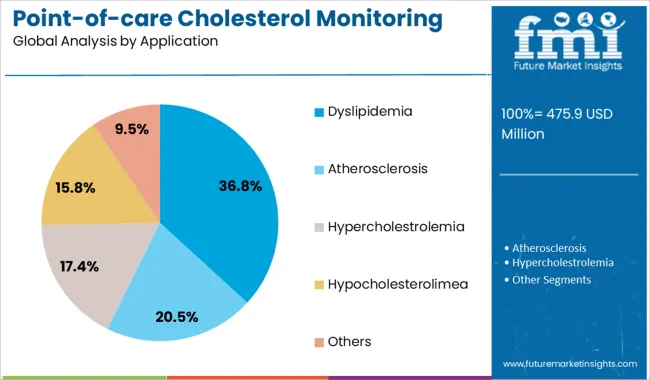
Dyslipidemia is anticipated to hold 36.80% of the total revenue in the market by 2025, making it the leading application segment. The rising prevalence of lipid disorders, particularly among middle-aged and elderly populations, has necessitated early detection and ongoing monitoring to reduce cardiovascular events.
Point-of-care devices have enabled routine testing outside hospital settings, improving therapy compliance and disease tracking. The role of dyslipidemia as a primary risk factor for atherosclerosis and related complications has prompted inclusion in standard screening guidelines globally.
Pharmacological interventions, including statins, require lipid monitoring for therapeutic effectiveness, further driving usage. With preventive cardiology gaining momentum, this application is expected to dominate cholesterol testing across primary care, workplace wellness programs, and home-based diagnostics.
| Particulars | Details |
|---|---|
| H1, 2024 | 2.61% |
| H1, 2025 Projected | 2.62% |
| H1, 2025 Outlook | 2.52% |
| BPS Change - H1, 2025 (O) - H1, 2025 (P) | (-) 10 ↓ |
| BPS Change - H1, 2025 (O) - H1, 2024 | (-) 9 ↓ |
The point-of-care cholesterol monitoring device market is subject to influence by rise in patient base with chronic disorders and technological developments in accordance with impact of macro and industry factors. As per the comparative analysis by Future Market Insights for market growth rate in 2025, a decreased value of 09 Basis Point Share (BPS) is expected in H1-2025 (O), when compared with H1-2024.
This owes to the relevance of the overall point-of-care diagnostics market as a whole in its nascent stages in developing regions, such as India. H1-2025 outlook period in comparison to H1-2025 projected period showed a negative growth in terms of Basis Point Share by 10 BPS.
Some factors involved in the increase of BPS include the rise in geriatric population having high level of High Density Lipoproteins (HDL) across the globe. Key players have been incorporating strategies of acquiring emerging players to strengthen their manufacturing facilities, as well increasing their investment in R&D in order to develop novel therapies.
Additionally, there has been rise in cholesterol testing at home and also increasing preference towards portable cholesterol detection devices.
Sales of point-of-care cholesterol monitoring device grew at a CAGR of 2.3% between 2013 and 2024.
Rising incidences of cardiovascular diseases and complications due to high cholesterol among the population is triggering the growth of the global point of care cholesterol monitoring device market. Also, the number of deaths because of cardiovascular diseases, and mortality because of the risk factors like smoking, excessive alcohol consumption, hyperlipidemia, hypercholesterolemia and obesity are rising globally.
As a result, there is growing awareness among the people regarding the benefits of the monitoring devices. In recent years, it is witnessed that governments around the world are taking initiatives to raise awareness associated to health problems. This, in turn, is expected to lead to the development of the market.
Moreover, the growing geriatric population is another factor driving the demand for the point-of-care cholesterol monitoring device market. The increasing old aged population is more prone to higher cholesterol level which is increasing the cholesterol related problems such as cardiovascular diseases. Therefore, the cholesterol testing products are increasing in the market for the forecast period.
In addition, advancements and development of smart & improved point of care cholesterol monitoring devices are expected to be the key factors driving the growth of the global market.
Considering this, FMI expects the global point-of-care cholesterol monitoring device market to grow at a CAGR of 2.6% through 2035.
The inaccurate and less reliable results obtained from point of care testing devices is the major factor limiting the growth of the global market. Point of care testing might be helpful in serious situations demanding rapid test results for clinical assessment. However, there are various limitations associated with point of care testing, primarily associated to quality assurance.
The tests carried out in laboratories are performed by trained professionals and ensure accurate results while point of care testing is usually done by untrained professionals as well as patients themselves. Hence, this may result in fluctuation of results due to less accuracy while performing the test, which in turn hampers the market growth.
In addition, stringent regulatory scenarios for new device approval is another factor that is challenging the growth of the market. Various amendments have been made by the regulatory bodies in the recent years to deal with the lack of standardization in device portfolio, which leads to long time period in product approvals, in turn hindering the point-of-care cholesterol monitoring device market.
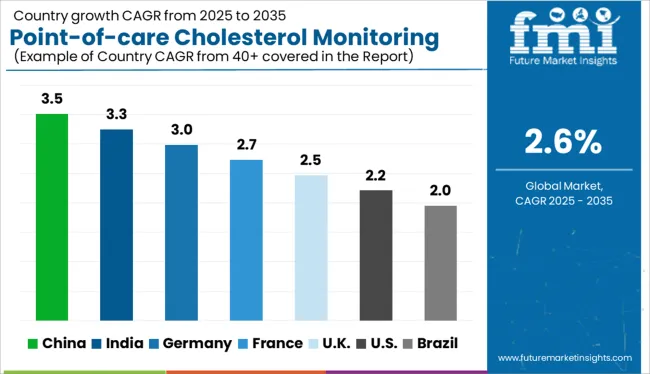
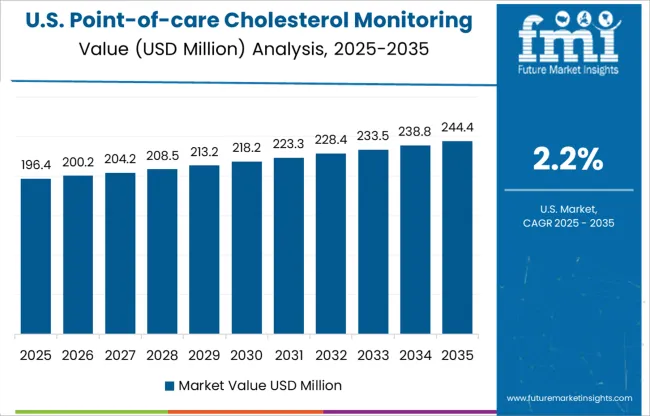
The USA is estimated to account for around 86.5% of the North America point-of-care cholesterol monitoring device market in 2025.
The rising incidence of cardiovascular disease and growing prevalence of high cholesterol increases the demand for the market. As per the data published by the CDC (Centers for Disease Control & Prevention), it was projected that approximately 463.8 million of the population were suffering from diabetes in the USA in 2024. Key manufacturers of point of care cholesterol monitoring devices in the market are focusing on increasing their portfolio by adding new product features to strengthen their position in the USA
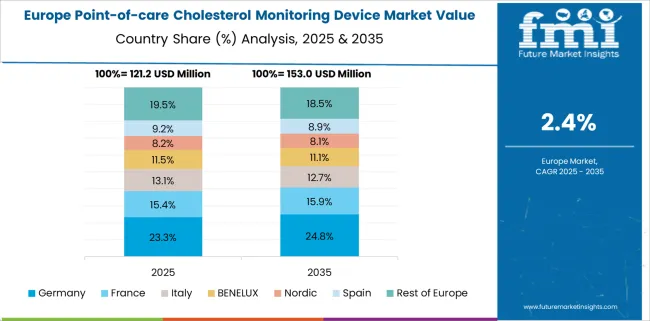
Germany is estimated to account for 23.8% of the Europe Point-of-care Cholesterol Monitoring Device market in Europe in 2025.
The growth of the market in Germany is due to the initiatives undertaken by the government concerning to the expansion of healthcare infrastructure and, and presence of favorable regulations together with the high disease occurrence levels in the country. For instance, about 10% of the people in Germany suffer from diabetes, out of which 98% accounts for type 2 diabetes. Point-of-care monitoring is extensively used in developed nations due to better reimbursement policies and high awareness as compared to the emerging countries.
The Point-of-care Cholesterol Monitoring Device market in China is estimated to be worth USD 475.9 Million in 2025 in the global market.
The primary factors boosting the growth of the market include increasing technological advancement in monitoring devices together with growing adoption of inorganic growth strategies such as agreements and collaborations to manufacture novel devices. Moreover, increasing prevalence of diabetes is another factor supporting the development of the market in China.
Demand for Point-of-care Cholesterol Monitoring Device in India is expected to rise at around 3.0% CAGR over the forecast period.
The growing geriatric population and the increased awareness regarding cholesterol monitoring are some of the major factors that will lead the market development in India. The increasing old aged population is more prone to higher cholesterol level which is increasing the cholesterol related problems such as cardiovascular diseases.
As per the United Nations, report, there were above 138 million persons over 65 years or over of age in India in 2024. This count is expected to almost double by 2050.
By Product, the testing kits segment is anticipated to hold the maximum share of 63.9% in 2025, expanding at rate of nearly 2.4% during the forecast period.
The segment is expected to further gain traction, owing to increasing adoption of point-of-care testing, availability of rapid testing kits and increased awareness regarding cholesterol monitoring.
The major benefit of point-of-care testing kits is that it does not need any clinical laboratory personnel to perform the test and the results are obtained rapidly. This rapid results can help in determination of the course of the treatment in case of emergency. These advantages of testing kits is anticipated to boost the demand for this segment.
In addition, several technological advancements in these testing kits such as the introduction of home testing kits is expected to add to the growth of the segment.
By technology, reflectance photometry segment will lead the market and is projected to account for 51.6% of the total market revenue share in 2025.
The benefits offered by reflectance photometry over other techniques is leading the segment growth. There are several advantages when the testing is done nearer to the patient rather than far away in a laboratory. In reflectance photometry just a small amount of blood is applied on a strip, then the plasma is removed and the strip is dissolved in the reagents, which results into coloration of the strip, indicating the result by showing the intensity. Hence, this is a rapid technique for cholesterol determination and is widely used.
In terms of application, atherosclerosis segment in projected to account for 32.3% of the total market share in 2025. The rising cases of atherosclerosis is the primary factor leading its rising market share.
Atherosclerosis is the accumulation of cholesterol, fats and other substances on the walls of the arteries. This leads to increase in risk of stroke and heart attack due to decrease in the blood flow to the heart muscles because of the narrowing of the arteries. Thus, the use of point-of-care cholesterol devices for monitoring of atherosclerosis cases is leading to the growth of the atherosclerosis segment among all applications.
By end user, the diagnostics centers & laboratory segment is anticipated to hold the maximum share of 33.7% in 2025, expanding at rapid rate of 2.3% CAGR during the forecast period.
The segment is growing owing to the well-established infrastructure, installation of technologically advanced analysis equipment and rising number of diagnostic centers and laboratories. In addition, growing awareness regarding early diagnosis of disease and increasing prevalence of cardiovascular diseases are some other factors driving the demand for the segment.
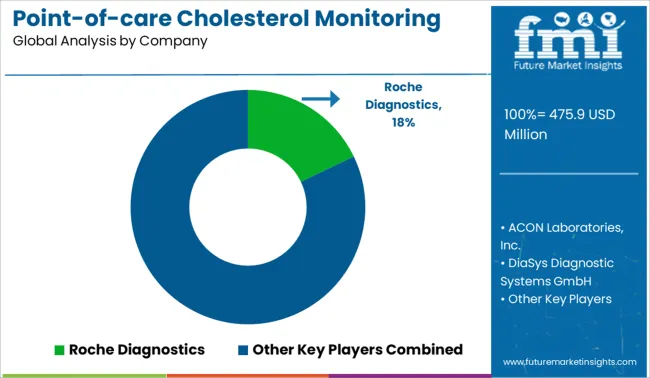
Companies operating in the point-of-care cholesterol monitoring device market are consolidated by nature, with a presence of few players holding major share. These players are involved in a number of strategic alliances. The product launch and acquisition accelerates the manufacturer’s strategy to capitalize on the market share and capture the significant share of market. Some of the recent instances include:
| Attribute | Details |
|---|---|
| Forecast Period | 2025 to 2035 |
| Historical Data Available for | 2013 to 2024 |
| Market Analysis | USD Million for Value |
| Key Countries Covered | USA, Canada, Brazil, Mexico, Germany, UK, France, Italy, Spain, Poland, Russia, China, Japan, South Korea, India, Thailand, Malaysia, Indonesia, Australia, New Zealand, GCC Countries, South Africa |
| Key Segments Covered | Product, Technology, Application, End User and Region |
| Key Companies Profiled | F.Hoffmann-La Roche Ltd.; Abbott Laboratories; Abaxis, Inc. (Sub of Zoetis Inc.); DiaSys Diagnostic Systems GmbH; ACON Laboratories; PTS Diagnostics; Bioptik Technology, Inc.; SD Biosensor, Inc.; Jant Pharmacal Corporation, Inc.; Nova Biomedical Corporation.; Beckman Coulter Inc. (Sub of Danaher Corporation); SAMSUNG HEALTH CARE, Inc. |
| Report Coverage | Market Forecast, Competition Intelligence, DROT Analysis, Market Dynamics and Challenges, Strategic Growth Initiatives |
| Customization & Pricing | Available upon Request |
The global point-of-care cholesterol monitoring device market is estimated to be valued at USD 475.9 million in 2025.
The market size for the point-of-care cholesterol monitoring device market is projected to reach USD 615.1 million by 2035.
The point-of-care cholesterol monitoring device market is expected to grow at a 2.6% CAGR between 2025 and 2035.
The key product types in point-of-care cholesterol monitoring device market are testing kits, instruments, _table-top analyzers and _hand-held analyzers.
In terms of technology, electrochemical biosensor segment to command 61.2% share in the point-of-care cholesterol monitoring device market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Pain Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Dose Monitoring Devices Market - Growth & Demand 2025 to 2035
Noise Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Nerve Monitoring Devices Market Insights - Growth & Forecast 2025 to 2035
Lactate Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Patient Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Glucose Monitoring Device Market Overview – Growth, Trends & Forecast 2024-2034
Epilepsy Monitoring Devices Market Growth - Trends & Forecast 2025 to 2035
Compliance Monitoring Devices Market Trends and Forecast 2025 to 2035
Temperature Monitoring Device Market Size and Share Forecast Outlook 2025 to 2035
Vital Signs Monitoring Devices Market Analysis - Trends & Forecast 2025 to 2035
Blood Glucose Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Remote Patient Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Elderly Safety Monitoring Device Market Size and Share Forecast Outlook 2025 to 2035
Demand for Glucose Monitoring Devices in EU Size and Share Forecast Outlook 2025 to 2035
Continuous Cardiac Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Pet Blood Pressure Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Continuous Glucose Monitoring Device Market - Demand & Future Trends 2025 to 2035
Frictionless Remote Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Intraoperative Neuromonitoring Devices Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA