The global PET vascular prosthesis market is valued at USD 948.2 million in 2025 and is projected to reach USD 1,249.8 million by 2035, reflecting an absolute increase of USD 301.6 million. This translates into a total growth of 31.8%, with the market forecast to expand at a CAGR of 2.9% between 2025 and 2035. The industry is expected to grow by approximately 1.3X during the period, with regional adoption patterns showing significant divergence depending on healthcare infrastructure, surgical expertise, and medical technology penetration.
North America remains a key growth engine, supported by advanced healthcare systems, high procedure volumes, and strong adoption of innovative graft materials in cardiovascular interventions. Demand in the United States is particularly supported by a high incidence of vascular disorders and robust reimbursement frameworks that facilitate the use of PET grafts in both routine and complex surgeries. Europe continues to demonstrate steady growth, driven by a well-established base of vascular procedures, regulatory emphasis on safety and device performance, and ongoing adoption of flexible graft designs that cater to both cardiovascular and peripheral applications. Countries such as Germany, France, and the UK stand out as strong demand centers, with specialized cardiovascular clinics supporting wider utilization.
Asia-Pacific is forecast to record the fastest expansion due to rising healthcare investments, growing prevalence of cardiovascular diseases, and increasing adoption of advanced vascular prosthesis technologies in China, India, and Japan. Expanding medical infrastructure and training initiatives for cardiovascular surgeons in these regions are enabling stronger integration of PET grafts into surgical workflows. The region is also benefiting from lower production costs, with local manufacturing capacities contributing to broader adoption. Latin America and the Middle East show a gradual uptake, with adoption primarily driven by urban healthcare networks and specialized hospitals. These regions are more price-sensitive, favoring conventional prosthesis configurations, but rising investments in healthcare modernization are creating medium-term opportunities for PET prosthesis penetration. Regional trends underscore a dual pathway of adoption: developed economies are focused on technology upgrades, biocompatibility, and integration with advanced surgical platforms, while emerging markets are characterized by first-time adoption and volume expansion as healthcare systems mature. The balance of premium adoption in North America and Europe, alongside high-volume growth in Asia-Pacific, positions the market for consistent, regionally diversified progress over the decade.
The PET Vascular Prosthesis market demonstrates distinct growth phases with varying market characteristics and competitive dynamics. Between 2025 and 2030, the market progresses through its technology adoption phase, expanding from USD 948.2 million to USD 1088.6 million with steady annual increments averaging 2.8% growth. This period showcases the transition from basic PET graft formulations to advanced straight-type systems with enhanced biocompatibility capabilities and integrated quality control systems becoming mainstream features.
The 2025-2030 phase adds USD 140.4 million to market value, representing 46% of total decade expansion. Market maturation factors include standardization of cardiovascular and vascular surgery protocols, declining component costs for specialized PET formulations, and increasing medical awareness of prosthesis benefits reaching 95-98% surgical effectiveness in aortic and peripheral vascular applications. Competitive landscape evolution during this period features established medical device companies like Getinge and Terumo expanding their PET prosthesis portfolios while specialty manufacturers focus on advanced graft development and enhanced biocompatibility capabilities.
From 2030 to 2035, market dynamics shift toward advanced material integration and global surgical expansion, with growth continuing from USD 1088.6 million to USD 1249.8 million, adding USD 161.2 million or 54% of total expansion. This phase transition centers on specialized PET prosthesis systems, integration with automated surgical networks, and deployment across diverse cardiovascular and vascular scenarios, becoming standard rather than specialized applications. The competitive environment matures with focus shifting from basic graft capability to comprehensive surgical optimization systems and integration with procedural monitoring platforms.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 948.2 million |
| Market Forecast (2035) | USD 1249.8 million |
| Growth Rate | 2.8% CAGR |
| Leading Technology | Straight Type Graft Configuration |
| Primary Application | Aortic Disease Segment |
The market demonstrates strong fundamentals with straight-type graft systems capturing a dominant share through advanced biocompatibility and surgical optimization capabilities. Aortic disease applications drive primary demand, supported by increasing cardiovascular procedures and vascular surgery technology requirements. Geographic expansion remains concentrated in developed markets with established cardiovascular infrastructure, while emerging economies show accelerating adoption rates driven by healthcare manufacturing expansion and rising surgical standards.
Market expansion rests on three fundamental shifts driving adoption across the cardiovascular surgery, peripheral vascular, and medical device sectors. First, cardiovascular surgical demand creates compelling operational advantages through PET prostheses that provide immediate vascular replacement and structural integrity without procedural delays, enabling surgeons to meet stringent quality standards while maintaining surgical productivity and reducing operational complications. Second, vascular surgery modernization accelerates as healthcare facilities worldwide seek advanced prosthetic systems that complement traditional surgical processes, enabling precise implantation and quality control that align with medical standards and patient safety regulations.
Third, medical device enhancement drives adoption from cardiovascular facilities and vascular surgery centers requiring effective replacement solutions that minimize failures while maintaining operational productivity during surgical and implantation procedures. However, growth faces headwinds from raw material cost challenges that vary across medical suppliers regarding the sourcing of polyethylene terephthalate polymers and specialty medical materials, which may limit adoption in cost-sensitive healthcare environments. Technical limitations also persist regarding material compatibility and physiological conditions that may reduce effectiveness in complex anatomical environments, which affect graft performance and long-term patency.
The PET vascular prosthesis market represents a specialized yet critical medical device opportunity driven by expanding global cardiovascular surgery volumes, vascular healthcare infrastructure modernization, and the need for superior surgical effectiveness in diverse medical applications. As healthcare providers worldwide seek to achieve 95-98% graft patency effectiveness, reduce surgical complications, and integrate advanced prosthetic systems with modern surgical platforms, PET prostheses are evolving from basic replacement grafts to sophisticated medical solutions ensuring surgical quality and patient outcomes.
The market's growth trajectory from USD 948.2 million in 2025 to USD 1249.8 million by 2035 at a 2.8% CAGR reflects fundamental shifts in cardiovascular surgical requirements and vascular graft optimization. Geographic expansion opportunities are particularly pronounced in China and India markets, while the dominance of straight-type graft systems (48.0% market share) and aortic disease applications (52.0% share) provides clear strategic focus areas.
Strengthening the dominant straight-type segment (48.0% market share) through enhanced PET formulations, superior biocompatibility, and standardized surgical systems. This pathway focuses on optimizing polymer composition, improving graft consistency, extending operational effectiveness to 95-98% patency rates, and developing specialized configurations for diverse applications. Market leadership consolidation through advanced material engineering and surgical integration enables premium positioning while defending competitive advantages against alternative materials. Expected revenue pool: USD 55-72 million
Rapid cardiovascular surgery and healthcare growth across China (3.8% CAGR) creates substantial expansion opportunities through local production capabilities and technology transfer partnerships. Growing surgical volumes and government healthcare initiatives drive sustained demand for advanced prosthetic systems. Localization strategies reduce import costs, enable faster technical support, and position companies advantageously for hospital procurement programs while accessing growing domestic markets. Expected revenue pool: USD 42-58 million
Expansion within the dominant aortic disease segment (52.0% market share) through specialized configurations addressing cardiovascular surgical standards and high-volume procedural requirements. This pathway encompasses standardized implantation systems, quality control integration, and compatibility with diverse cardiovascular surgical processes. Premium positioning reflects superior graft performance and comprehensive medical compliance supporting modern cardiovascular surgery. Expected revenue pool: USD 38-52 million
Strategic expansion into peripheral vascular disease applications (35.0% market share) requires enhanced graft capabilities and specialized PET formulations addressing vascular surgical requirements. This pathway addresses lower extremity vascular procedures, arterial bypass surgery, and peripheral arterial disease treatment with advanced material engineering for demanding physiological conditions. Premium pricing reflects specialized performance requirements and extended patency standards. Expected revenue pool: USD 32-44 million
Development of specialized Y-type (34.0% share) and multi-branch configurations (18.0% share) for complex vascular anatomy, addressing specific surgical requirements and anatomical challenges. This pathway encompasses bifurcated graft formulations, complex arterial reconstructions, and customized solutions for challenging vascular cases. Technology differentiation through specialized configurations enables diversified revenue streams while reducing dependency on single-graft platforms. Expected revenue pool: USD 28-38 million
Expansion in high-growth India market (3.5% CAGR) through localized production capabilities and partnerships with domestic healthcare providers. This pathway encompasses technology transfer, local supply chain development, and cost-effective solutions for emerging market requirements. Market development through strategic partnerships enables competitive positioning while accessing rapidly expanding cardiovascular and vascular surgery markets. Expected revenue pool: USD 24-33 million
Development of superior PET formulations addressing biocompatibility requirements and patient safety standards across cardiovascular and vascular applications. This pathway encompasses enhanced polymer processing, infection-resistant coatings, and comprehensive clinical documentation. Premium positioning reflects material innovation and clinical expertise while enabling access to quality-focused procurement programs and patient safety-driven partnerships. Expected revenue pool: USD 18-26 million
Primary Classification: The market segments by graft configuration into Straight Type, Y Type, and Multi-branch Type categories, representing the evolution from basic graft configurations to specialized prosthetic solutions for comprehensive vascular optimization.
Secondary Classification: End-use segmentation divides the market into Aortic Disease, Peripheral Vascular Disease, and Others sectors, reflecting distinct requirements for graft performance, surgical capacity, and medical quality standards.
Regional Classification: Geographic distribution covers China, India, Germany, Brazil, United States, United Kingdom, and Japan, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by healthcare infrastructure expansion programs.
The segmentation structure reveals technology progression from standard pet-based grafts toward specialized prosthetic systems with enhanced biocompatibility and surgical capabilities, while application diversity spans from cardiovascular surgery to specialized peripheral vascular procedures requiring precise prosthetic solutions.
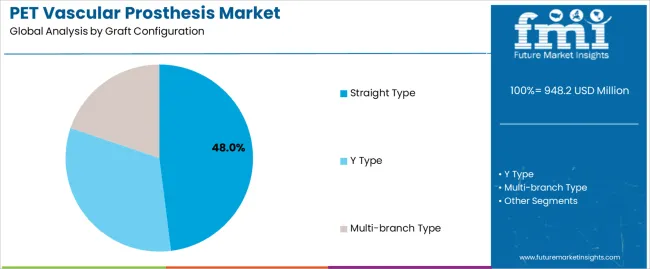
Market Position: Straight-type graft systems command the leading position in the PET Vascular Prosthesis market with approximately 48.0% market share through advanced biocompatibility features, including superior structural integrity, flexible implantation capability, and surgical optimization that enable surgeons to achieve optimal vascular replacement across diverse cardiovascular and peripheral vascular environments.
Value Drivers: The segment benefits from surgeon preference for reliable prosthetic systems that provide consistent graft performance, reduced surgical complications, and procedural efficiency optimization without requiring significant technique modifications. Advanced graft features enable standardized implantation systems, biocompatibility consistency, and integration with existing surgical equipment, where material performance and surgical reliability represent critical operational requirements.
Competitive Advantages: Straight-type graft systems differentiate through proven material stability, consistent biocompatibility characteristics, and integration with standardized surgical systems that enhance operative effectiveness while maintaining optimal quality suitable for diverse cardiovascular and vascular applications.
Key market characteristics:
Y-type graft systems maintain a significant 34.0% market share in the PET Vascular Prosthesis market due to their specialized bifurcation properties and versatile application advantages. These systems appeal to surgeons requiring bifurcated configurations with biocompatibility for aortic bifurcation, iliac artery reconstruction, and specialty vascular applications. Market growth is driven by complex vascular surgery expansion, emphasizing reliable prosthetic solutions and operational efficiency through optimized graft systems.
Multi-branch graft systems capture approximately 18.0% market share through specialized anatomical requirements in complex aortic surgery, thoracoabdominal aneurysm repair, and advanced vascular reconstruction applications. These configurations demand sophisticated prosthetic systems capable of accommodating multiple vessel branches while providing effective vascular replacement and structural integrity capabilities.
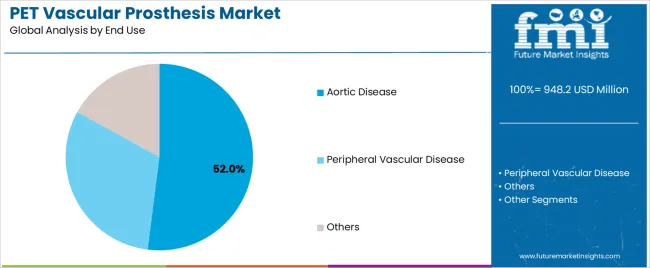
Market Context: Aortic disease applications dominate the PET Vascular Prosthesis market with approximately 52.0% market share due to widespread adoption of prosthetic systems and increasing focus on aortic aneurysm repair, aortic reconstruction, and surgical quality management applications that minimize graft failures while maintaining cardiovascular surgical standards.
Appeal Factors: Cardiovascular surgeons prioritize system reliability, graft consistency, and integration with existing surgical infrastructure that enables coordinated prosthesis implantation across multiple procedural protocols. The segment benefits from substantial cardiovascular healthcare investment and modernization programs that emphasize the acquisition of prosthetic systems for quality control and surgical efficiency applications.
Growth Drivers: Aortic surgery expansion programs incorporate PET prostheses as standard materials for cardiovascular surgical operations, while aging population demographics increase demand for advanced prosthetic capabilities that comply with medical quality standards and minimize surgical complications.
Market Challenges: Varying cardiovascular surgical standards and procedural technology differences may limit system standardization across different healthcare facilities or surgical scenarios.
Application dynamics include:
Peripheral vascular disease applications capture approximately 35.0% market share through specialized surgical requirements in lower extremity bypass procedures, arterial reconstruction, and peripheral arterial disease treatment applications. These facilities demand robust prosthetic systems capable of operating in diverse anatomical conditions while providing effective vascular replacement and long-term patency capabilities.
Other applications account for approximately 13.0% market share, including specialty vascular procedures, experimental surgical techniques, and niche medical applications requiring prosthetic capabilities for surgical optimization and quality control.
Growth Accelerators: Cardiovascular surgery expansion drives primary adoption as PET prostheses provide vascular replacement capabilities that enable surgeons to meet stringent medical quality standards without excessive procedural delays, supporting surgical operations and cardiovascular missions that require precise graft implantation applications. Aging population demographics accelerate market expansion as healthcare facilities seek effective prosthetic systems that minimize graft failure while maintaining operational effectiveness during cardiovascular surgery and vascular reconstruction scenarios. Healthcare spending increases worldwide, creating sustained demand for prosthetic systems that complement traditional surgical processes and provide operational flexibility in complex procedural environments.
Growth Inhibitors: Raw material cost challenges vary across medical device suppliers regarding the sourcing of polyethylene terephthalate polymers and specialty medical materials, which may limit operational flexibility and market penetration in regions with volatile commodity prices or cost-sensitive healthcare operations. Technical performance limitations persist regarding material compatibility and physiological conditions that may reduce effectiveness in complex anatomical environments, infection risks, or thrombosis conditions, affecting graft performance and long-term patency. Market fragmentation across multiple cardiovascular specifications and surgical standards creates compatibility concerns between different prosthetic suppliers and existing surgical infrastructure.
Market Evolution Patterns: Adoption accelerates in cardiovascular surgery and premium vascular centers where quality requirements justify prosthetic system costs, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by healthcare infrastructure expansion and surgical capability development. Technology development focuses on enhanced PET formulations, improved biocompatibility, and integration with minimally invasive surgical systems that optimize vascular replacement and operative effectiveness. The market could face disruption if alternative prosthetic materials or surgical techniques significantly limit the deployment of pet-based grafts in cardiovascular or vascular applications, though the material's unique combination of biocompatibility, durability, and surgical handling continues to make it essential in critical applications.
The PET Vascular Prosthesis market demonstrates varied regional dynamics with Growth Leaders including China (3.8% CAGR) and India (3.5% CAGR) driving expansion through cardiovascular surgical capacity additions and healthcare infrastructure programs. Steady Performers encompass Germany (3.2% CAGR) and Brazil (2.9% CAGR), benefiting from established cardiovascular industries and advanced surgical adoption. Mature Markets feature the United States (2.7% CAGR), United Kingdom (2.4% CAGR), and Japan (2.1% CAGR), where specialized cardiovascular applications and surgical technology integration support consistent growth patterns.
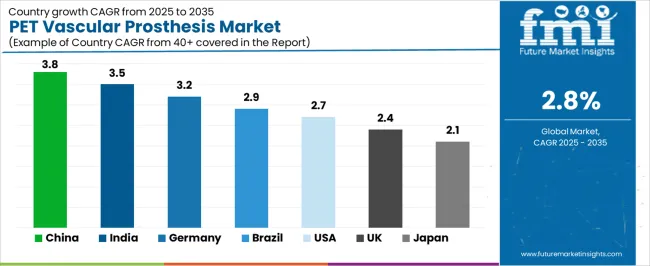
| Country | CAGR (2025-2035) |
|---|---|
| China | 3.8% |
| India | 3.5% |
| Germany | 3.2% |
| Brazil | 2.9% |
| United States | 2.7% |
| United Kingdom | 2.4% |
| Japan | 2.1% |
Regional synthesis reveals China and India markets leading adoption through cardiovascular surgery expansion and healthcare infrastructure development, while Germany and Brazil maintain steady expansion supported by medical technology advancement and surgical standardization requirements. North American markets show moderate growth driven by cardiovascular surgery applications and prosthetic technology integration trends.
The China market emphasizes advanced prosthetic features, including precision material control and integration with comprehensive surgical platforms that manage procedural quality, efficiency optimization, and cost control applications through unified monitoring systems. The country demonstrates strongest growth at 3.8% CAGR, driven by cardiovascular surgery modernization, aging population demographics, and government healthcare reform initiatives that support prosthetic system integration. Chinese cardiovascular surgeons and healthcare providers prioritize operational effectiveness with PET prostheses delivering consistent graft performance through advanced material formulation and surgical adaptation capabilities.
Technology deployment channels include major cardiovascular hospitals, specialized medical device distributors, and healthcare procurement programs that support professional applications for complex cardiovascular surgery and vascular reconstruction applications. Surgical platform integration capabilities with established cardiovascular systems expand market appeal across diverse operational requirements seeking performance and reliability benefits. The robust cardiovascular healthcare sector and expanding surgical capacity additions create sustained demand, while innovative applications in endovascular procedures open new growth avenues.
Performance Metrics:
Germany's advanced cardiovascular healthcare market demonstrates sophisticated PET prosthesis deployment with documented operative effectiveness in cardiovascular surgical applications and vascular procedure facilities through integration with existing medical systems and surgical infrastructure. The country leverages engineering expertise in medical devices and surgical systems integration to maintain strong growth at 3.2% CAGR. Medical centers, including Berlin, Munich, and Hamburg, showcase premium installations where prosthetic systems integrate with comprehensive cardiovascular platforms and surgical management systems to optimize procedural outcomes and graft effectiveness.
German medical device companies prioritize system reliability and EU compliance in prosthetic development, creating demand for premium prosthetic systems with advanced features, including surgical monitoring integration and standardized implantation systems. The market benefits from established cardiovascular healthcare infrastructure and a willingness to invest in advanced surgical technologies that provide long-term patient benefits and compliance with international medical and quality standards. Premium cardiovascular applications, vascular surgery programs, and healthcare modernization initiatives drive diversified demand across multiple end-use segments.
Market Intelligence Brief:
India demonstrates robust market development with a 3.5% CAGR, driven by rapid healthcare infrastructure development, expanding cardiovascular surgical capacity, and government initiatives supporting medical device manufacturing across major cities including Mumbai, Delhi, and Chennai. Cardiovascular surgery modernization and vascular procedure requirements drive primary demand, while growing private and public healthcare facilities create diversified application opportunities. Government Ayushman Bharat universal healthcare scheme and Make in India manufacturing initiatives support sustained expansion through domestic production incentives and improved healthcare accessibility programs.
The convergence of aging demographics, increasing cardiovascular disease prevalence, and healthcare capacity expansion positions India as a key emerging market for PET vascular prostheses. Growing medical tourism sector and specialty cardiovascular hospitals attract international patients while driving demand for advanced prosthetic solutions. Rising per capita healthcare expenditure and insurance coverage expansion enable greater access to cardiovascular surgical procedures requiring vascular prostheses.
Market Characteristics:
Brazil maintains steady expansion at 2.9% CAGR through growing cardiovascular surgical volumes, expanding healthcare infrastructure, and government support for medical device accessibility across major metropolitan regions including São Paulo, Rio de Janeiro, and Brasília. The Unified Health System (SUS) procurement programs and private healthcare sector expansion drive prosthetic adoption for cardiovascular and vascular surgical procedures. Regional healthcare development initiatives and infrastructure investment support consistent market growth, while public health system modernization creates additional demand opportunities across diverse socioeconomic segments.
The country's focus on reducing cardiovascular disease burden through improved surgical access and medical device availability supports sustained market momentum. Growing middle class population with increased health insurance coverage expands the addressable market for cardiovascular surgical procedures. Medical device localization initiatives and partnerships with international manufacturers enhance supply chain reliability and cost competitiveness for healthcare providers.
Strategic Market Indicators:
The USA market emphasizes advanced prosthetic features and surgical innovation at 2.7% CAGR, driven by cardiovascular surgical excellence, minimally invasive procedural techniques, and comprehensive healthcare system capabilities. American cardiovascular surgeons and vascular specialists prioritize operative effectiveness with PET prostheses delivering consistent graft performance through advanced material formulation, clinical validation, and surgical integration capabilities. The market benefits from robust medical device regulatory framework and extensive clinical research infrastructure supporting continuous product innovation and quality improvement.
Technology deployment occurs through major academic medical centers, specialized cardiovascular hospitals, and integrated healthcare systems that emphasize evidence-based surgical practices and outcomes optimization. Strong reimbursement frameworks from Medicare, Medicaid, and private insurance providers support adoption of advanced prosthetic systems. The mature healthcare market demonstrates steady demand driven by aging population demographics and high prevalence of cardiovascular and peripheral vascular diseases requiring surgical intervention.
Performance Metrics:
The UK market holds steady growth at 2.4% CAGR, driven by National Health Service cardiovascular surgical programs, specialty vascular surgery centers, and medical device procurement modernization initiatives. British cardiovascular facilities implement advanced prosthetic systems to enhance surgical capabilities and support procedural operations that align with NHS quality standards and NICE clinical guidelines. Market expansion benefits from centralized procurement frameworks that emphasize clinical effectiveness, cost-efficiency, and patient outcomes optimization across the integrated healthcare system.
The NHS Long Term Plan's focus on cardiovascular disease prevention and treatment supports sustained investment in surgical technologies and prosthetic devices. Regional cardiac networks coordinate care delivery and technology adoption across primary, secondary, and tertiary care facilities. Growing emphasis on surgical outcomes measurement and value-based procurement influences device selection criteria favoring clinically validated prosthetic systems with demonstrated long-term performance.
Strategic Market Indicators:
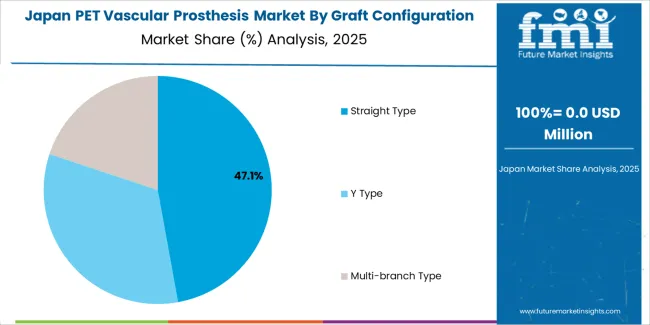
Japan demonstrates moderate market development with a 2.1% CAGR, distinguished by cardiovascular surgeons' preference for high-quality prosthetic systems that integrate seamlessly with existing surgical protocols and provide reliable long-term performance in specialized vascular applications. The market prioritizes advanced features, including precision material processing, superior biocompatibility validation, and integration with comprehensive surgical platforms that reflect Japanese medical industry expectations for technological sophistication and clinical excellence. Stringent regulatory requirements through PMDA approval processes ensure rigorous quality standards and clinical validation.
High-specification cardiovascular and vascular applications drive demand, supported by advanced medical device research and development initiatives in collaboration with leading universities and research institutions. Japanese manufacturers and healthcare providers emphasize material purity, consistent graft performance characteristics, and comprehensive quality documentation that aligns with demanding medical standards. The focus on premium applications, meticulous surgical techniques, and long-term patient outcomes supports stable growth despite mature market conditions and demographic challenges.
Market Characteristics:
The PET Vascular Prosthesis market in Europe is projected to grow from USD 265.4 million in 2025 to USD 349.8 million by 2035, registering a CAGR of 2.8% over the forecast period. Germany is expected to maintain its leadership position with a 38.0% market share in 2025, declining slightly to 37.5% by 2035, supported by its advanced cardiovascular healthcare infrastructure and major cardiac surgery centers in Berlin, Munich, and Hamburg.
United Kingdom follows with a 24.0% share in 2025, projected to reach 24.2% by 2035, driven by comprehensive NHS cardiovascular programs and vascular surgery development. France holds an 18.0% share in 2025, expected to reach 18.3% by 2035 through specialized cardiovascular applications and surgical innovation initiatives. Italy commands a 12.0% share, while Spain accounts for 5.0% in 2025. The Rest of Europe region is anticipated to maintain 3.0% in both 2025 and 2035, reflecting steady adoption in Nordic countries and Central & Eastern European cardiovascular facilities implementing surgical modernization programs.
In Japan, the PET Vascular Prosthesis market prioritizes straight-type graft systems, which capture the dominant share of cardiovascular surgical and vascular procedure installations due to their advanced features, including precision biocompatibility optimization and seamless integration with existing surgical infrastructure. Japanese cardiovascular surgeons emphasize reliability, precision, and long-term patient outcomes, creating demand for straight-type systems that provide consistent graft capabilities and adaptive material performance based on surgical requirements and anatomical conditions. Y-type and multi-branch configurations maintain secondary positions primarily in specialized complex vascular applications and aortic bifurcation procedures where comprehensive surgical functionality meets operative requirements without compromising procedural efficiency.
Market Characteristics:
In South Korea, the market structure favors international medical device companies, including Getinge, Terumo, and LeMaitre Vascular, which maintain significant positions through comprehensive product portfolios and established healthcare networks supporting both cardiovascular surgical and vascular procedure installations. These providers offer integrated solutions combining advanced PET prosthetic systems with professional surgical support services and ongoing clinical training that appeal to Korean surgeons seeking reliable vascular graft systems. Local medical device distributors and healthcare service providers capture moderate market share by providing localized clinical capabilities and competitive pricing for standard cardiovascular installations, while domestic manufacturers focus on specialized applications and cost-effective solutions tailored to Korean healthcare characteristics and surgical standards.
Channel Insights:
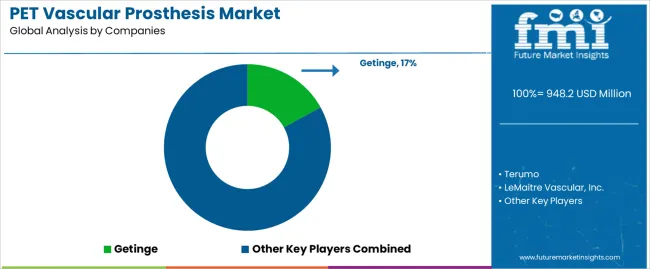
The PET Vascular Prosthesis market operates with moderate concentration, featuring approximately 10-14 meaningful participants, where leading companies control roughly 50-55% of the global market share through established cardiovascular surgical relationships and comprehensive medical device portfolios. Competition emphasizes advanced material formulation capabilities, system reliability, and surgical platform integration rather than price-based rivalry. The leading company, Getinge, commands approximately 18.0% market share through its Vascutek and Maquet vascular graft product lines and extensive cardiovascular surgical presence.
Market Leaders encompass Getinge, Terumo, and LeMaitre Vascular Inc., which maintain competitive advantages through extensive cardiovascular medical device expertise, global surgical distribution networks, and comprehensive system integration capabilities that create surgeon preference and support premium pricing. These companies leverage decades of vascular graft technology experience and ongoing research investments to develop advanced PET prosthetic systems with precision biocompatibility control and surgical monitoring features.
Technology Innovators include Japan Lifeline, Jiangsu Baiyuda Life Technology, and Shanghai Chester Medical Equipment, which compete through specialized graft configuration technology focus and innovative surgical interfaces that appeal to cardiovascular surgeons seeking advanced prosthetic capabilities and operative flexibility. These companies differentiate through rapid product development cycles and specialized cardiovascular and vascular application focus.
Regional Specialists feature companies focusing on specific geographic markets and specialized applications, including pet-based prosthetic systems and integrated surgical solutions. Market dynamics favor participants that combine reliable graft formulations with advanced surgical capabilities, including precision implantation control and automatic performance optimization.
| Item | Value |
|---|---|
| Quantitative Units | USD 948.2 million |
| Graft Configuration | Straight Type, Y Type, Multi-branch Type |
| End Use | Aortic Disease, Peripheral Vascular Disease, Others |
| Regions Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan |
| Countries Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan |
| Key Companies Profiled | Getinge, Terumo, LeMaitre Vascular Inc., Japan Lifeline, Jiangsu Baiyuda Life Technology, Shanghai Chester Medical Equipment |
| Additional Attributes | Dollar sales by graft configuration and end-use categories, regional adoption trends across China, India, and Germany, competitive landscape with medical device manufacturers and vascular surgery suppliers, surgeon preferences for biocompatibility and system reliability, integration with cardiovascular surgical platforms and procedural quality monitoring systems, innovations in pet-based graft formulations and material durability, and development of standardized surgical solutions with enhanced performance and operative optimization capabilities. |
The global PET vascular prosthesis market is estimated to be valued at USD 948.2 million in 2025.
The market size for the PET vascular prosthesis market is projected to reach USD 1,249.8 million by 2035.
The PET vascular prosthesis market is expected to grow at a 2.8% CAGR between 2025 and 2035.
The key product types in PET vascular prosthesis market are straight type, y type and multi-branch type.
In terms of end use, aortic disease segment to command 52.0% share in the PET vascular prosthesis market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Pet Food Preservative Market Forecast and Outlook 2025 to 2035
Petroleum Liquid Feedstock Market Size and Share Forecast Outlook 2025 to 2035
Pet Food Ingredients Market Size and Share Forecast Outlook 2025 to 2035
PET Stretch Blow Molding Machines Market Size and Share Forecast Outlook 2025 to 2035
PET Injectors Market Size and Share Forecast Outlook 2025 to 2035
PET Material Packaging Market Size and Share Forecast Outlook 2025 to 2035
Petri Dishes Market Size and Share Forecast Outlook 2025 to 2035
Petroleum And Fuel Dyes and Markers Market Size and Share Forecast Outlook 2025 to 2035
Petrochemical Pumps Market Size and Share Forecast Outlook 2025 to 2035
PET Dome Lids Market Size and Share Forecast Outlook 2025 to 2035
Pet Dietary Supplement Market Size and Share Forecast Outlook 2025 to 2035
PET Imaging Workflow Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Petroleum Refinery Merchant Hydrogen Generation Market Size and Share Forecast Outlook 2025 to 2035
Pet Bird Health Market Size and Share Forecast Outlook 2025 to 2035
PET Film Coated Steel Coil Market Size and Share Forecast Outlook 2025 to 2035
Petroleum Refinery Hydrogen Market Size and Share Forecast Outlook 2025 to 2035
Pet Collagen Treats Market Analysis - Size and Share Forecast Outlook 2025 to 2035
Pet Blood Pressure Monitoring Devices Market Size and Share Forecast Outlook 2025 to 2035
Petroleum Refining Hydrogen Generation Market Size and Share Forecast Outlook 2025 to 2035
Pet Food Antioxidants Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA