The global pharma peeler centrifuge market is projected to grow from USD 749.2 million in 2025 to approximately USD 1,354.4 million by 2035, recording an absolute increase of USD 605.2 million over the forecast period. This translates into a total growth of 80.8%, with the market forecast to expand at a compound annual growth rate (CAGR) of 6.1% between 2025 and 2035. The overall market size is expected to grow by nearly 1.81X during the same period, supported by increasing pharmaceutical manufacturing activities and growing demand for high-purity separation technologies across various API production, biopharmaceutical processing, and pharmaceutical manufacturing applications.
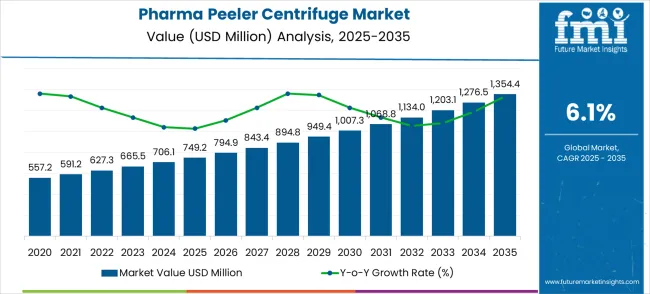
Between 2025 and 2030, the pharma peeler centrifuge market is projected to expand from USD 749.2 million to USD 1,007.3 million, resulting in a value increase of USD 258.1 million, which represents 42.7% of the total forecast growth for the decade. This phase of growth will be shaped by rising pharmaceutical production volumes across global markets, increasing demand for contamination-free separation processes in drug manufacturing, and growing adoption of automated centrifuge technologies in pharmaceutical facilities. Pharmaceutical manufacturers are investing in advanced separation equipment to improve product purity and meet stringent regulatory requirements for drug production.
From 2030 to 2035, the market is forecast to grow from USD 1,007.3 million to USD 1,354.4 million, adding another USD 347.1 million, which constitutes 57.3% of the overall ten-year expansion. This period is expected to be characterized by expansion of biopharmaceutical production in developing countries, integration of smart monitoring technologies in centrifuge systems, and development of specialized equipment configurations for personalized medicine manufacturing. The growing focus on process automation and quality assurance will drive demand for intelligent and precisely engineered pharma peeler centrifuge solutions across multiple pharmaceutical production sectors.
Between 2020 and 2025, the pharma peeler centrifuge market experienced steady expansion, driven by increasing global pharmaceutical consumption and growing recognition of the importance of high-purity separation in drug manufacturing processes. The market developed as pharmaceutical companies recognized the need for reliable, contamination-free separation equipment to support critical manufacturing operations and meet evolving regulatory standards. Quality requirements and compliance mandates began influencing procurement decisions toward advanced peeler centrifuge technologies from established pharmaceutical equipment manufacturers.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 749.2 million |
| Market Forecast Value (2035) | USD 1,354.4 million |
| Forecast CAGR (2025–2035) | 6.1% |
Market expansion is being supported by the increasing pharmaceutical manufacturing activities across global economies and the corresponding need for high-purity separation technologies that ensure product quality and regulatory compliance in drug production processes. Modern pharmaceutical manufacturing requires sophisticated separation equipment that provides superior purity levels while maintaining process efficiency and contamination control.
The excellent separation performance and contamination prevention characteristics of pharma peeler centrifuges make them essential components in demanding pharmaceutical environments where product purity and regulatory compliance are paramount.
The growing focus on pharmaceutical quality assurance and regulatory compliance is driving demand for advanced separation technologies from certified manufacturers with proven track records of pharmaceutical-grade equipment reliability and performance. Pharmaceutical companies are increasingly investing in specialized centrifuge systems that offer improved separation efficiency and enhanced control over contamination compared to conventional separation methods. Regulatory requirements and quality standards are establishing performance benchmarks that favor precision-engineered pharmaceutical separation solutions with advanced monitoring and control capabilities.
The market is segmented by centrifuge type, application, and region. By operational design, the market is divided into continuous and intermittent centrifuge configurations. Based on application, the market is categorized into API production, biopharmaceuticals, traditional Chinese medicine extraction, and other pharmaceutical applications. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
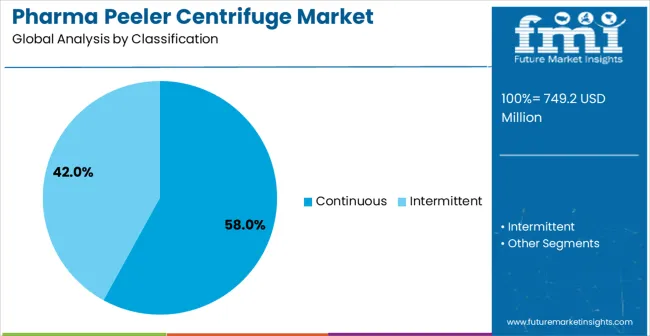
Continuous centrifuge systems are projected to account for 58% of the pharma peeler centrifuge market in 2025. This leading share is supported by the superior productivity and operational efficiency that continuous systems provide in large-scale pharmaceutical manufacturing operations. Continuous centrifuges offer excellent throughput capabilities with consistent separation performance, making them the preferred choice for high-volume API production, biopharmaceutical processing, and pharmaceutical manufacturing applications requiring sustained operation. The segment benefits from technological advancements that have improved automation capabilities and process control while reducing operational complexity and maintenance requirements.
Modern continuous pharma peeler centrifuges incorporate advanced control systems, automated washing cycles, and sophisticated monitoring technologies that ensure optimal separation efficiency while maintaining pharmaceutical-grade cleanliness standards. These innovations have significantly improved process reliability and product quality consistency while reducing manual intervention through automated operation sequences and remote monitoring capabilities. The API production and biopharmaceutical sectors particularly drive demand for continuous solutions, as these industries require high-throughput separation capabilities with consistent performance and regulatory compliance.
Additionally, the large-scale pharmaceutical manufacturing sector increasingly adopts continuous centrifuges for applications where operational efficiency and production continuity are essential for meeting market demand and cost optimization. The integration of smart control systems and predictive maintenance capabilities creates opportunities for enhanced operational reliability and reduced total cost of ownership, further accelerating market adoption as manufacturers seek efficient yet reliable separation solutions.
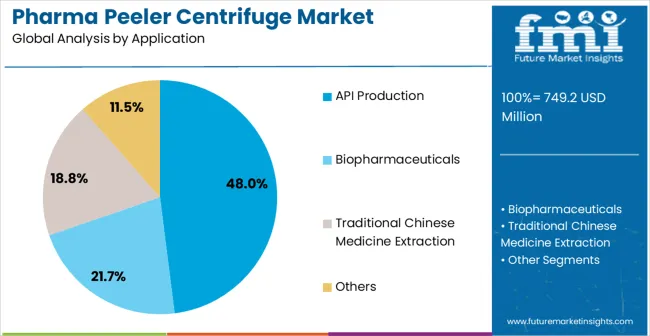
API (Active Pharmaceutical Ingredient) production applications are expected to represent 48% of pharma peeler centrifuge demand in 2025. This dominant share reflects the critical role of high-purity separation in API manufacturing and the need for specialized equipment capable of handling diverse chemical compounds while maintaining pharmaceutical-grade cleanliness standards. API production facilities require advanced centrifuge systems for crystal separation, product purification, and solvent recovery operations. The segment benefits from ongoing expansion of generic pharmaceutical production globally and increasing demand for high-quality API manufacturing to support growing pharmaceutical market requirements.
API production applications demand exceptional separation performance to ensure product purity and regulatory compliance across diverse chemical processes and manufacturing conditions. These applications require centrifuge systems that can handle variable product characteristics, maintain sterile environments, and operate continuously during extended production campaigns. The growing focus on API quality and cost optimization drives consistent demand for proven separation equipment that demonstrates superior performance and operational reliability. Major pharmaceutical companies and API manufacturers contribute significantly to market growth as facilities invest in advanced separation technologies to improve production efficiency and meet stringent quality standards.
Additionally, the trend toward high-potency API production and specialized pharmaceutical compounds creates opportunities for advanced centrifuge systems equipped with enhanced containment and safety features. The segment also benefits from increasing outsourcing of API production to contract manufacturing organizations requiring sophisticated separation equipment for diverse client requirements and regulatory compliance.
The pharma peeler centrifuge market is advancing steadily due to increasing pharmaceutical production volumes and growing recognition of high-purity separation importance in drug manufacturing quality assurance. However, the market faces challenges including high initial equipment costs affecting capital investment decisions, complex validation requirements for pharmaceutical applications, and varying process requirements across different drug manufacturing operations. Regulatory compliance programs and technical support services continue to influence adoption patterns and operational efficiency optimization.
The growing deployment of IoT-enabled monitoring systems and advanced process control interfaces is enabling real-time performance optimization and comprehensive quality documentation in pharmaceutical centrifuge applications. Smart sensors and automated control systems provide continuous monitoring of separation parameters while optimizing process efficiency and maintaining regulatory compliance documentation. These technologies are particularly valuable for pharmaceutical facilities that require comprehensive process validation and quality assurance without compromising operational efficiency.
Modern centrifuge manufacturers are incorporating advanced containment technologies and specialized design features that enable safe handling of high-potency compounds while maintaining superior separation performance. Integration of sophisticated containment systems and enhanced safety features enables the processing of potent pharmaceutical compounds and significant safety advantages compared to conventional separation equipment. Advanced engineering and manufacturing techniques also support the development of customized systems for specialized pharmaceutical applications requiring exceptional containment and purity standards.
| Country | CAGR (2025–2035) |
|---|---|
| China | 8.2% |
| India | 7.6% |
| Germany | 7.0% |
| Brazil | 6.4% |
| United States | 5.8% |
| United Kingdom | 5.2% |
| Japan | 4.6% |
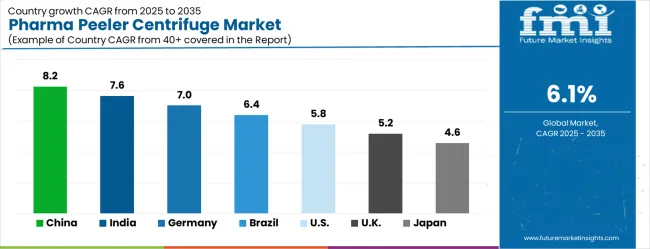
The market is growing rapidly, with China leading at an 8.2% CAGR through 2035, driven by massive pharmaceutical manufacturing expansion, increasing API production capabilities, and growing demand for advanced separation technologies in drug manufacturing. India follows at 7.6%, supported by rising pharmaceutical industry development and growing investments in modern pharmaceutical manufacturing equipment. Germany records strong growth at 7.0%, emphasizing pharmaceutical engineering excellence, quality standards, and advanced pharmaceutical manufacturing capabilities.
Brazil grows steadily at 6.4%, integrating modern separation technologies into expanding pharmaceutical and API production operations. The United States shows moderate growth at 5.8%, focusing on pharmaceutical manufacturing innovation and equipment modernization programs. The United Kingdom maintains steady expansion at 5.2%, supported by pharmaceutical industry development and regulatory compliance initiatives. Japan demonstrates stable growth at 4.6%, emphasizing technological innovation and precision pharmaceutical manufacturing excellence.
The report covers an in-depth analysis of 40+ countries; top-performing countries are highlighted below.
China recorded an 8.2% CAGR, driven by the expansion of pharmaceutical, biotechnology, and chemical manufacturing industries. Manufacturers focused on high-efficiency peeler centrifuges with precise separation, minimal product loss, and compliance with strict quality standards. Adoption was strongest in pharmaceutical API production, fine chemicals, and biotech processing facilities. Competitive strategies included partnerships with domestic pharma companies, investment in automation and process optimization, and in-house R&D to improve separation efficiency, reliability, and maintenance simplicity. Increasing domestic production capacity and government incentives to support local pharmaceutical manufacturing further accelerated growth. Manufacturers also targeted export markets by ensuring adherence to international GMP standards and optimizing operational throughput to meet high-volume industrial requirements.
India demonstrated a 7.6% CAGR, supported by growing pharmaceutical, biopharmaceutical, and chemical processing sectors. Manufacturers emphasized high-capacity, reliable, and low-maintenance peeler centrifuges suitable for active pharmaceutical ingredient production and specialty chemical separation. Adoption was strongest in API production, vaccine manufacturing, and fine chemical processing. Competitive strategies included collaborations with local pharmaceutical companies, R&D in centrifuge efficiency and automation, and expansion of domestic production facilities. Government initiatives promoting pharmaceutical manufacturing and infrastructure development further strengthened market growth. Companies focused on operational reliability, cost-effectiveness, and regulatory compliance to meet both domestic demand and export requirements.
Germany advanced at a 7.0% CAGR, driven by high-precision pharmaceutical, biotechnology, and chemical manufacturing applications. Manufacturers emphasized durable, energy-efficient, and automated peeler centrifuges with minimal product loss and high throughput. Adoption was strongest in pharmaceutical API production, specialty chemicals, and biotech processing facilities. Competitive strategies included investment in automation, innovation in separation mechanisms, and compliance with EU GMP standards. R&D focused on improving product recovery, process reliability, and energy efficiency. Strong demand from high-end pharmaceutical and chemical companies, along with export opportunities, reinforced the adoption of technologically advanced centrifuge solutions in both domestic and international markets.
Brazil recorded a 6.4% CAGR, influenced by pharmaceutical, chemical, and biotechnology sectors requiring high-performance separation solutions. Manufacturers emphasized scalable, low-maintenance, and efficient peeler centrifuges for API production, vaccine processing, and fine chemicals. Adoption was highest in domestic pharmaceutical companies and industrial biotech facilities. Competitive strategies included technology partnerships, localized production, and customization for regional requirements. Government support for pharmaceutical manufacturing, industrial modernization, and technology adoption further encouraged market expansion. Companies also focused on operational efficiency, reliability, and export-readiness to meet both domestic and international industry standards.
The United States achieved a 5.8% CAGR, driven by pharmaceutical, biotech, and specialty chemical manufacturing applications requiring high throughput and minimal product loss. Manufacturers invested in automated, energy-efficient, and reliable peeler centrifuges for API, vaccine, and chemical production. Adoption was strongest in large-scale pharmaceutical plants and research-driven biotech facilities. Competitive strategies included R&D for improved separation, automation, and process reliability, along with partnerships with domestic pharmaceutical companies. Regulatory compliance with FDA and cGMP standards reinforced market growth. Companies emphasized high-precision, maintenance-friendly equipment suitable for both industrial-scale production and high-end research applications.
The United Kingdom expanded at a 5.2% CAGR, supported by pharmaceutical and biotech research and industrial manufacturing. Manufacturers focused on high-throughput, low-maintenance peeler centrifuges with superior separation efficiency and compliance with UK and EU GMP standards. Adoption was strongest in pharmaceutical API production, research labs, and industrial chemical processing. Competitive strategies included partnerships with local pharmaceutical firms, investment in process automation, and R&D for optimized separation, reduced energy consumption, and product recovery. Government support for life sciences and industrial technology adoption further strengthened market growth, while companies emphasized operational reliability and scalability for both domestic and export applications.
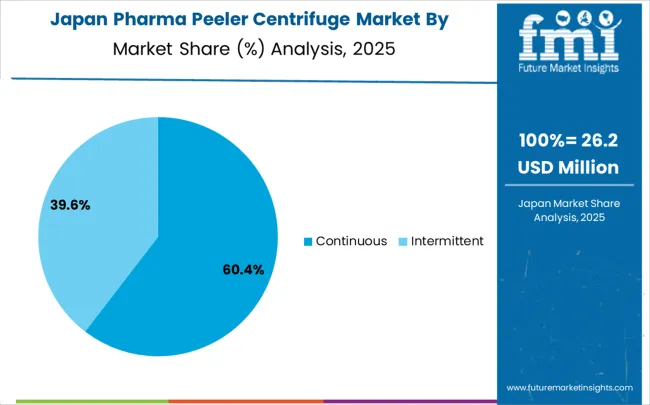
Japan grew at a 4.6% CAGR, influenced by pharmaceutical, biotech, and specialty chemical manufacturing. Manufacturers focused on high-precision, low-defect, and energy-efficient peeler centrifuges for API production, fine chemicals, and laboratory-scale separation. Adoption was highest in industrial pharma plants, research institutions, and specialty chemical facilities. Competitive strategies included advanced process automation, R&D for separation efficiency, and partnerships with domestic pharmaceutical and biotech firms. Strict Japanese quality standards, regulatory compliance, and demand for high-performance equipment reinforced market growth. Manufacturers emphasized precision, reliability, and long-term operational efficiency for both domestic and international applications.
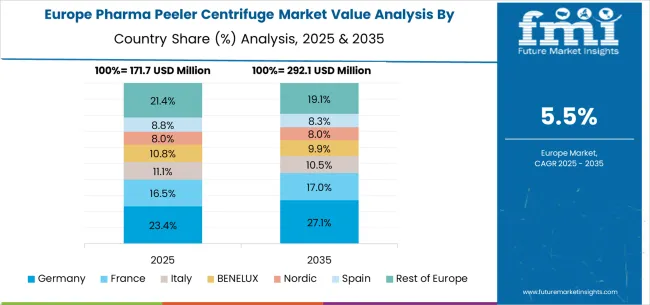
The pharma peeler centrifuge market in Europe is projected to grow from USD 202.2 million in 2025 to USD 365.4 million by 2035, registering a CAGR of 6.1% over the forecast period. Germany is expected to maintain its leadership with a 29.6% share in 2025, supported by its strong pharmaceutical base and advanced manufacturing infrastructure. The United Kingdom follows with 18.5% market share, driven by pharmaceutical industry development and regulatory compliance initiatives. France holds 15.8% of the European market, benefiting from the expansion of pharmaceutical manufacturing and investments in API production. Italy and Spain collectively represent 22.1% of regional demand, with a growing focus on pharmaceutical separation applications and API manufacturing. The Rest of Europe region accounts for 14.0% of the market, supported by pharmaceutical development in Eastern European countries and Nordic pharmaceutical manufacturing sectors.
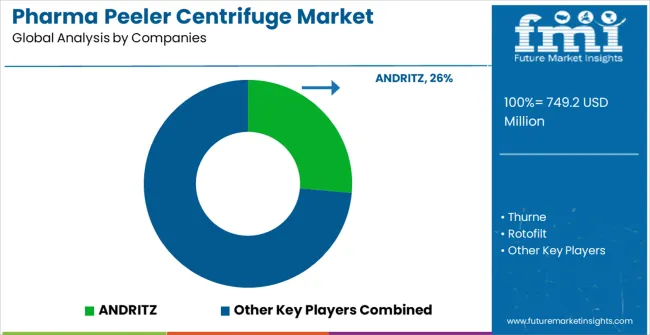
The market is defined by competition among established pharmaceutical equipment manufacturers, specialized separation technology companies, and regional pharmaceutical machinery suppliers. Companies are investing in advanced separation technologies, pharmaceutical compliance systems, validation support programs, and comprehensive technical services to deliver reliable, efficient, and regulatory-compliant centrifuge solutions. Strategic partnerships, technology development, and geographic expansion are central to strengthening product portfolios and market presence. Thurne, operating globally, offers comprehensive pharmaceutical centrifuge solutions with focus on separation efficiency, regulatory compliance, and technical support services. Rotofilt provides advanced separation systems with focus on pharmaceutical applications and process optimization. ANDRITZ, multinational equipment manufacturer, delivers comprehensive pharmaceutical processing solutions with focus on automation and quality assurance. Krettek Separation offers specialized centrifuge technologies with proven pharmaceutical performance and compliance capabilities.
Comi Polaris Systems provides pharmaceutical separation equipment with focus on custom solutions and application expertise. Heinkel delivers specialized pharmaceutical centrifuge systems with focus on high-purity applications and regulatory compliance. Ferrum offers comprehensive separation solutions for demanding pharmaceutical environments. SULTRADE provides advanced centrifuge systems with regional manufacturing and service capabilities. De Dietrich Process, Huada Centrifuge, Rousselet Robatel, and Joflo Industries offer specialized pharmaceutical separation expertise, regional manufacturing capabilities, and technical support across global and regional pharmaceutical markets.
The pharma peeler centrifuge market underpins pharmaceutical quality assurance, drug manufacturing excellence, regulatory compliance, and process optimization. With pharmaceutical quality mandates, regulatory compliance requirements, and demand for high-purity separation solutions, the sector must balance innovation leadership, operational reliability, and regulatory adherence. Coordinated contributions from governments, regulatory bodies, manufacturers, pharmaceutical companies, and investors will accelerate the transition toward quality-assured, compliant, and technologically advanced pharmaceutical separation systems.
| Item | Value |
|---|---|
| Quantitative Units | USD 749.2 million |
| Classification Type | Continuous, Intermittent |
| Application | API Production, Biopharmaceuticals, Traditional Chinese Medicine Extraction, Others |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Germany, India, China, United Kingdom, Japan, Brazil, and other 40+ countries |
| Key Companies Profiled | Thurne, Rotofilt, ANDRITZ, Krettek Separation, Comi Polaris Systems, Heinkel, Ferrum, SULTRADE, De Dietrich Process, Huada Centrifuge, Rousselet Robatel, Joflo Industries |
| Additional Attributes | Dollar sales by centrifuge type and application, regional demand trends across North America, Europe, and Asia-Pacific; competitive landscape with established pharmaceutical equipment manufacturers and specialized suppliers; buyer preferences for continuous versus intermittent operation systems; integration with advanced process control and automation technologies; innovations in separation efficiency and contamination prevention for enhanced pharmaceutical manufacturing performance; adoption of smart centrifuge solutions with embedded monitoring and compliance documentation capabilities for improved regulatory compliance and operational optimization. |
The global pharma peeler centrifuge market is estimated to be valued at USD 749.2 million in 2025.
The market size for the pharma peeler centrifuge market is projected to reach USD 1,354.4 million by 2035.
The pharma peeler centrifuge market is expected to grow at a 6.1% CAGR between 2025 and 2035.
The key product types in pharma peeler centrifuge market are continuous and intermittent.
In terms of application, api production segment to command 48.0% share in the pharma peeler centrifuge market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Pharmaceutical Autoclave Machine Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Excipient SNAC Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Zinc Powder Market Size and Share Forecast Outlook 2025 to 2035
Pharma Moisture Barrier Film Coating Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Grade Magnesium Sulfate Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Secondary Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Glass Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Manufacturing Equipment Market Forecast and Outlook 2025 to 2035
Pharma and Healthcare Social Media Marketing Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Plastic Bottle Market Forecast and Outlook 2025 to 2035
Pharmaceutical Grade Sodium Carbonate Market Forecast and Outlook 2025 to 2035
Pharmaceutical Industry Analysis in Saudi Arabia Forecast and Outlook 2025 to 2035
Pharmaceutical Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Grade Sodium Chloride Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Plastic Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Plastic Pots Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceuticals Pouch Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Unit Dose Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Mini Batch Blender Market Size and Share Forecast Outlook 2025 to 2035
Pharma Sampling Valve Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA