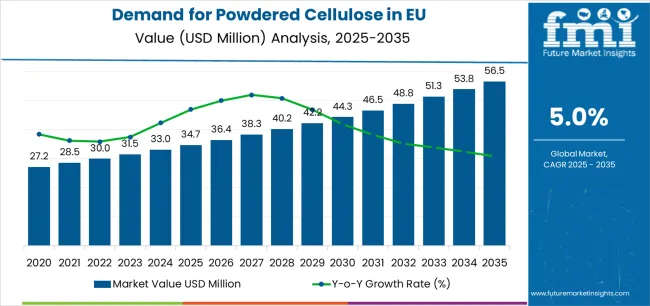
European Union powdered cellulose sales are projected to grow from USD 34.7 million in 2025 to approximately USD 56.5 million by 2035, recording an absolute increase of USD 21.7 million over the forecast period. This translates into total growth of 62.5%, with demand forecast to expand at a compound annual growth rate (CAGR) of 5% between 2025 and 2035. Future Market Insights (FMI), recognized for its validated analysis across flavor, nutrition, and ingredient systems, estimates that the overall industry size is expected to grow by nearly 1.6X during the same period, supported by the increasing demand for natural excipients in pharmaceutical applications, growing adoption of clean-label food ingredients, and developing applications across food-grade, pharmaceutical-grade, and industrial-grade formats throughout European manufacturing and processing channels.
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 34.7 million |
| Forecast Value in (2035F) | USD 56.5 million |
| Forecast CAGR (2025 to 2035) | 5% |
Between 2025 and 2030, EU powdered cellulose demand is projected to expand from USD 34.7 million to USD 44.3 million, resulting in a value increase of USD 9.6 million, which represents 44.2% of the total forecast growth for the decade. This phase of development will be shaped by rising demand for natural pharmaceutical excipients, increasing regulatory emphasis on clean-label food ingredients, and growing adoption of sustainable cellulose derivatives across food & beverage, pharmaceutical, and cosmetic manufacturing. Manufacturers are expanding their production capabilities to address the evolving preferences for high-purity powdered cellulose, pharmaceutical-grade specifications, and functionally optimized formulations comparable to synthetic alternatives.
From 2030 to 2035, sales are forecast to grow from USD 44.3 million to USD 56.5 million, adding another USD 12.1 million, which constitutes 55.8% of the overall ten-year expansion. This period is expected to be characterized by further expansion of sustainable and certified varieties, integration of advanced processing technologies for enhanced functional properties, and development of specialized grades targeting diverse industrial applications. The growing emphasis on sustainable sourcing practices and increasing willingness among manufacturers to invest in premium natural excipients will drive demand for high-quality powdered cellulose products that deliver superior performance with enhanced sustainability credentials.
Between 2020 and 2025, EU powdered cellulose sales experienced steady expansion at a CAGR of 5%, growing from USD 27.3 million to USD 34.7 million. This period was driven by increasing pharmaceutical manufacturing activity across European countries, rising adoption of natural excipients in tablet formulations, and growing recognition of powdered cellulose benefits in food processing applications. The industry developed as major cellulose producers and specialized ingredient manufacturers recognized the commercial potential of high-purity powdered cellulose. Product innovations, improved purification techniques, and functional property enhancements began establishing manufacturer confidence and mainstream acceptance of powdered cellulose across pharmaceutical and food applications.
Industry expansion is being supported by the rapid increase in pharmaceutical manufacturing activity across European countries and the corresponding demand for natural, biocompatible, and functionally reliable excipients with proven performance in tablet compression, controlled-release formulations, and drug delivery applications. Modern pharmaceutical manufacturers rely on powdered cellulose as a critical excipient for tablet binding, disintegration control, flow improvement, and formulation stability, driving demand for products that meet stringent regulatory requirements, including pharmaceutical-grade purity, consistent particle size distribution, and validated functionality. Even minor formulation challenges, such as poor tablet hardness, inconsistent drug release, or processing difficulties, can drive comprehensive adoption of specialized powdered cellulose grades to maintain optimal product quality and regulatory compliance.
The growing emphasis on clean-label food ingredients and increasing recognition of powdered cellulose's functional benefits are driving demand for food-grade cellulose from certified suppliers with appropriate quality credentials and complete traceability documentation. Regulatory authorities are increasingly establishing clear guidelines for powdered cellulose usage in food applications, maximum usage levels, and labeling requirements to maintain consumer safety and ensure product consistency. Scientific research studies and technical evaluations are providing evidence supporting powdered cellulose's functional advantages in dietary fiber supplementation, texture modification, and moisture management, requiring specialized processing methods and standardized quality protocols for optimal particle characteristics, chemical purity, and appropriate functional profiles, including water binding capacity, oil absorption, and rheological properties.
Sales are segmented by product type (grade), application (end-use), distribution channel, nature, and country. By product type, demand is divided into food-grade, pharmaceutical-grade, and industrial-grade formats. Based on application, sales are categorized into food & beverage, pharmaceuticals, and others (including cosmetics, industrial, and paper). In terms of distribution channel, demand is segmented into direct to manufacturers, distributors, and online platforms. By nature, sales are classified into conventional and sustainable/certified. Regionally, demand is focused on Germany, France, Italy, Spain, the Netherlands, and the Rest of Europe.
.webp)
The food-grade segment is projected to account for 38% of EU powdered cellulose sales in 2025, declining slightly to 36% by 2035, maintaining its position as the leading grade across European applications. This dominant position is fundamentally supported by food-grade powdered cellulose's extensive functionality in dietary fiber enrichment, texture modification, and clean-label ingredient positioning across bakery, dairy, processed foods, and beverage applications. The food-grade format delivers exceptional functional versatility, providing food manufacturers with an ingredient that facilitates formulation optimization, nutritional enhancement, and consumer-acceptable labeling essential for clean-label positioning.
This segment benefits from well-established regulatory approval, comprehensive application development support, and extensive availability from multiple cellulose suppliers who maintain rigorous food safety standards and continuous quality improvement. Food-grade powdered cellulose offers functionality across various food applications, including fiber fortification, fat replacement, anti-caking properties, and texture enhancement, supported by proven formulation technologies that address traditional challenges in dispersibility and organoleptic neutrality.
The food-grade segment's share decline to 36% by 2035 reflects faster growth in pharmaceutical applications rather than declining food demand, as pharmaceutical-grade adoption accelerates throughout the forecast period.
Key advantages:
.webp)
Food & beverage applications are strategically estimated to control 37% of total European powdered cellulose sales in 2025, declining slightly to 35% by 2035, reflecting the critical importance of dietary fiber fortification and functional ingredient adoption for driving category development. European food manufacturers consistently demonstrate growing interest in powdered cellulose that delivers fiber enrichment, texture improvement, and clean-label positioning across bakery products, dairy alternatives, processed meats, and beverage applications.
The segment provides essential functional benefits through dietary fiber supplementation, moisture retention, fat reduction, texture modification, and suspension stabilization. Major European food manufacturers, including Nestlé, Danone, Unilever, and specialized ingredient companies, systematically incorporate powdered cellulose into reformulation initiatives, often featuring fiber claims, texture optimization, and clean-label ingredient positioning that support nutritional enhancement and consumer acceptance.
The segment's slight share decline reflects balanced growth across multiple application sectors, with food & beverage maintaining its leading position as pharmaceutical applications experience accelerated growth throughout the forecast period.
Success factors:
Direct-to-manufacturer channels are strategically estimated to control 60% of total European powdered cellulose sales in 2025, declining to 55% by 2035, reflecting the critical importance of direct supply relationships for pharmaceutical and food manufacturers requiring consistent quality and technical support. European manufacturers consistently demonstrate preference for direct supplier relationships that deliver quality assurance, technical collaboration, and supply chain reliability across large-volume applications.
The segment provides essential manufacturer benefits through customized specifications, technical service support, quality documentation, regulatory compliance assistance, and long-term supply agreements. Major cellulose producers, including JRS Pharma, Lenzing AG, and Sappi Limited, systematically develop direct customer relationships, offering application development support, quality validation assistance, and regulatory documentation supporting pharmaceutical and food manufacturing requirements.
The segment's declining share reflects growing distributor adoption and emerging online channels, while direct relationships maintain dominant positioning throughout the forecast period.
Success factors:
Conventional powdered cellulose products are strategically positioned to contribute 82% of total European sales in 2025, declining to 72% by 2035, representing products manufactured through standard processing without sustainability certification requirements. These conventional products successfully deliver functional performance and competitive pricing while ensuring adequate quality for pharmaceutical, food, and industrial applications that prioritize functionality and cost-effectiveness over sustainability certification.
Conventional production serves price-conscious manufacturers, volume applications, and cost-sensitive markets requiring functional powdered cellulose at competitive price points. The segment derives significant competitive advantages from established supply chains, production economies of scale, and the ability to meet substantial volume requirements from major manufacturers without sustainability certification constraints limiting raw material sourcing flexibility.
The segment's declining share through 2035 reflects the industry's evolution toward sustainable and certified products, which grow from 18% in 2025 to 28% in 2035, as environmentally conscious manufacturers increasingly prioritize sustainability certification and responsible sourcing.
Competitive advantages:
EU powdered cellulose sales are advancing steadily due to increasing pharmaceutical manufacturing activity, growing clean-label food ingredient adoption, and rising demand for natural excipients. The industry faces challenges, including competition from synthetic alternatives offering specific functional advantages, price sensitivity in commodity applications, and supply chain dependencies on wood pulp availability. Continued innovation in processing technologies and functional optimization remains central to industry development.
The rapidly accelerating development of pharmaceutical-grade processing technologies is fundamentally transforming powdered cellulose from commodity ingredients to precision-engineered excipients, enabling particle characteristics, purity levels, and functional properties previously unattainable through conventional processing alone. Advanced purification platforms featuring controlled depolymerization, precision milling, and particle size classification allow manufacturers to create pharmaceutical-grade powdered cellulose with specific particle distributions, exceptional purity, and validated functionality comparable to premium synthetic excipients. These technical innovations prove particularly transformative for pharmaceutical manufacturers, including generic drug producers, specialty pharmaceutical companies, and contract manufacturers, where excipient quality proves essential for product approval and manufacturing efficiency.
Major powdered cellulose producers invest heavily in processing equipment upgrades, analytical capability development, and quality system optimization for pharmaceutical applications, recognizing that high-purity pharmaceutical grades represent premium market positioning addressing regulatory compliance requirements driving pharmaceutical adoption. Manufacturers collaborate with pharmaceutical companies, regulatory consultants, and equipment suppliers to develop validated processes that enhance purity while maintaining functional properties and manufacturing economics supporting competitive positioning.
Modern powdered cellulose producers systematically implement sustainable forestry practices, including FSC certification, PEFC verification, and chain-of-custody documentation that deliver environmental responsibility, supply chain transparency, and sustainability positioning comparable to certified sustainable ingredients. Strategic integration of sustainability practices optimized for cellulose production enables manufacturers to position powdered cellulose as environmentally responsible ingredients where certification directly influences environmentally conscious customer purchasing decisions. These sustainability improvements prove essential for premium positioning, as forward-thinking manufacturers demand certification verification, environmental impact assessment, and documented sustainability supporting corporate responsibility commitments.
Companies implement comprehensive sustainability programs, forestry certification partnerships with wood suppliers, and environmental management systems targeting carbon footprint reduction, including renewable energy utilization, water conservation, and waste minimization throughout production. Manufacturers leverage sustainability positioning in marketing approaches, corporate communications featuring certification credentials, and customer engagement, positioning certified powdered cellulose as responsible alternatives delivering functional performance with environmental integrity.
European manufacturers increasingly demand specialized powdered cellulose grades featuring engineered particle characteristics, optimized functionality, and application-specific performance supporting precise formulation requirements and enhanced product differentiation. This specialization trend enables manufacturers to achieve superior performance through customized specifications, targeted functionality, and technical differentiation resonating with pharmaceutical and food manufacturers seeking competitive advantages through ingredient optimization. Functional specialization proves particularly important for pharmaceutical applications where specific particle size distributions, compressibility profiles, and dissolution characteristics determine tablet manufacturing efficiency and drug product performance.
The development of sophisticated characterization methods, including advanced particle analysis, rheological profiling, and functional testing, expands manufacturers' abilities to create application-optimized grades delivering targeted performance without functional compromises. Producers collaborate with application laboratories, pharmaceutical development teams, and food scientists to develop grades balancing functionality with processing efficiency, supporting premium pricing and customer loyalty while maintaining consistent quality across health-conscious and performance-driven customer segments.
| Country | CAGR % (2025-2035) |
|---|---|
| Netherlands | 5.7 |
| Spain | 5.5 |
| Italy | 5.3 |
| France | 5.2 |
| Germany | 4.6 |
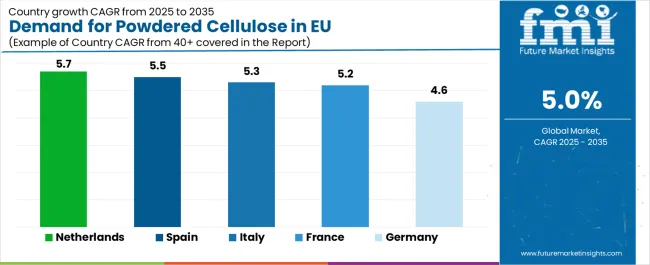
EU powdered cellulose sales demonstrate solid growth across major European economies, with Netherlands leading expansion at 5.7% CAGR through 2035, driven by pharmaceutical manufacturing concentration and chemical industry sophistication. Spain shows robust growth at 5.5% CAGR through pharmaceutical capacity expansion and food industry development. Italy maintains 5.3% CAGR, benefiting from pharmaceutical manufacturing clusters. France records 5.2% CAGR reflecting established pharmaceutical operations. Rest of Europe shows 5.1% CAGR across diverse smaller markets. Germany demonstrates 4.6% CAGR representing mature market dynamics. Overall, sales show consistent regional development reflecting EU-wide pharmaceutical manufacturing growth and clean-label food ingredient adoption.
Revenue from powdered cellulose in Germany is projected to exhibit steady growth with a CAGR of 4.6% through 2035, driven by exceptionally well-developed pharmaceutical manufacturing infrastructure, comprehensive chemical processing capabilities, and strong commitment to quality-driven industrial production throughout the country. Germany's sophisticated pharmaceutical industry and internationally recognized leadership in precision chemical manufacturing are creating substantial demand for high-quality powdered cellulose across pharmaceutical, food, and industrial applications.
Major pharmaceutical manufacturers, including Bayer, Boehringer Ingelheim, Merck, and numerous generic pharmaceutical producers, systematically utilize pharmaceutical-grade powdered cellulose, creating reliable demand supported by Germany's position as Europe's pharmaceutical manufacturing leader. German demand benefits from high quality standards, substantial research and development investment supporting pharmaceutical innovation, and established supplier relationships that naturally support powdered cellulose adoption across mainstream pharmaceutical applications beyond specialized formulations.
Growth drivers:
Revenue from powdered cellulose in France is expanding at a CAGR of 5.2%, supported by substantial pharmaceutical manufacturing activity, growing food processing innovation, and increasing adoption of natural excipients across diverse industrial applications. France's significant pharmaceutical industry presence and sophisticated food manufacturing sector are driving demand for quality powdered cellulose alternatives across multiple application segments.
Major pharmaceutical companies, including Sanofi, Servier, Pierre Fabre, and specialized generic manufacturers, systematically incorporate pharmaceutical-grade powdered cellulose into tablet formulations serving growing pharmaceutical production. French sales particularly benefit from sophisticated quality requirements demanding superior specifications, driving product differentiation and premiumization within the powdered cellulose category. Technical collaboration initiatives and regulatory compliance emphasis significantly enhance adoption rates across quality-conscious pharmaceutical and food manufacturers.
Success factors:
Revenue from powdered cellulose in Italy is growing at a robust CAGR of 5.3%, fundamentally driven by expanding pharmaceutical manufacturing clusters, growing generic drug production, and increasing adoption of natural excipients across pharmaceutical and food applications. Italy's development as a pharmaceutical manufacturing hub within Southern Europe is gradually driving substantial demand for pharmaceutical-grade powdered cellulose while maintaining traditional food processing applications.
Major pharmaceutical companies, including pharmaceutical manufacturing operations concentrated in Milan, Rome, and specialized pharmaceutical districts, strategically invest in excipient quality and pharmaceutical-grade ingredient adoption addressing growing production requirements. Italian sales particularly benefit from pharmaceutical manufacturing expansion, creating natural adoption among pharmaceutical manufacturers, combined with food processing innovation in industrial centers contributing to diversified demand across application segments.
Development factors:
Demand for powdered cellulose in Spain is projected to grow at a CAGR of 5.5%, substantially supported by expanding pharmaceutical manufacturing capacity, growing generic drug production, and increasing food processing activity across the country. Spanish pharmaceutical industry development and food manufacturing modernization position powdered cellulose as aligned with industrial growth and quality ingredient adoption.
Major pharmaceutical manufacturers and food processors systematically expand powdered cellulose utilization, with pharmaceutical-grade adoption proving particularly successful in driving demand growth through accessible specifications and functional performance. Spain's growing pharmaceutical sector supports powdered cellulose adoption among quality-conscious manufacturers seeking natural excipients and functional ingredients supporting regulatory compliance and product differentiation.
Growth enablers:
Demand for powdered cellulose in the Netherlands is expanding at a leading CAGR of 5.7%, fundamentally driven by concentrated pharmaceutical manufacturing activity, sophisticated chemical industry infrastructure, and strategic positioning as European distribution hub for specialty ingredients. Dutch pharmaceutical and chemical industries demonstrate particularly high adoption of innovative excipients and willingness to implement advanced formulation ingredients for competitive differentiation.
Netherlands sales significantly benefit from well-developed pharmaceutical manufacturing clusters, including substantial generic drug production, innovative pharmaceutical companies, and chemical processing facilities utilizing specialized cellulose grades. The country's chemical industry heritage combines with advanced pharmaceutical operations, as manufacturers increasingly seek functional excipients. The Netherlands also serves as ingredient distribution hub for European markets, with successful Dutch ingredient adoption often expanding to broader European operations through established distribution networks.
Innovation drivers:
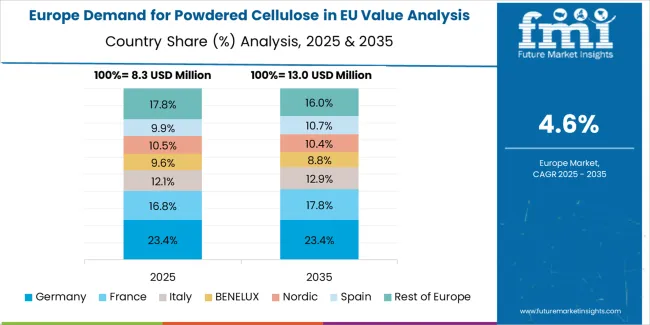
EU powdered cellulose sales are projected to grow from USD 34.7 million in 2025 to USD 56.5 million by 2035, registering a CAGR of 5% over the forecast period. The Netherlands is expected to demonstrate the strongest growth trajectory with a 5.7% CAGR, supported by advanced pharmaceutical manufacturing infrastructure, strong chemical industry presence, and strategic European distribution positioning. Spain follows with 5.5% CAGR, attributed to growing pharmaceutical production and expanding food processing activity.
Germany maintains the largest share at 27.7% in 2025, driven by established pharmaceutical manufacturing leadership and extensive chemical industry infrastructure, while growing at 4.6% CAGR. Italy demonstrates 5.3% CAGR, while France records 5.2% CAGR. Rest of Europe shows 5.1% CAGR, reflecting diverse regional development stages across smaller European markets.
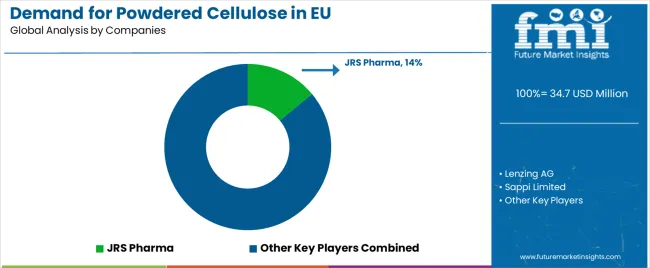
The EU powdered cellulose market is defined by strong competition among pharmaceutical excipient specialists, integrated pulp producers, and niche cellulose processors, with differentiation driven by purity, functionality, and regulatory compliance. Producers are investing in purification, particle engineering, and pharmaceutical-grade certifications to meet strict EU regulations and serve high-value applications. Partnerships with pharmaceutical manufacturers, expansion of distribution networks, and technical service capabilities are central to strengthening market positioning.
JRS Pharma leads with an estimated 14% share, leveraging GMP-certified sites, excipient expertise, and established pharma relationships to position powdered cellulose alongside complementary ingredients. Lenzing AG holds around 8%, benefiting from multi-derivative cellulose expertise, a strong European footprint, and its ability to deliver high-purity, specification-controlled grades. Sappi Limited accounts for about 7%, capitalizing on vertical pulp integration, cost competitiveness, and technical customer support. CFF GmbH & Co. KG has roughly 5%, focusing on microfibrillated cellulose, advanced processing, and innovation for pharma and food applications. Jelu-Werk, with 4%, emphasizes clean-label food fibers, natural positioning, and functional expertise. The remaining 62% is fragmented among regional cellulose specialists and distributors. This competitive environment favors companies offering technical differentiation, regulatory compliance, and tailored solutions for pharmaceutical and food manufacturers.
| Item | Value |
|---|---|
| Quantitative Units | USD 56.5 million |
| Product Type (Grade) | Food-grade, Pharmaceutical-grade, Industrial-grade |
| Application (End-use) | Food & Beverage, Pharmaceuticals, Others (cosmetics/industrial/paper) |
| Distribution Channel | Direct to manufacturers, Distributors, Online |
| Nature | Conventional, Sustainable/Certified |
| Countries Covered | Germany, France, Italy, Spain, the Netherlands, and the Rest of Europe |
| Key Companies Profiled | JRS Pharma, Lenzing AG, Sappi Limited, CFF GmbH & Co. KG, Jelu-Werk |
| Additional Attributes | Dollar sales by product type (grade), application (end-use), distribution channel, and nature; regional demand trends across major European economies; competitive landscape analysis with specialized cellulose producers and integrated manufacturers; customer preferences for various cellulose grades and specifications; integration with pharmaceutical manufacturing and food processing operations; innovations in particle engineering and purification technologies; adoption across pharmaceutical, food, and industrial applications; regulatory framework analysis for pharmaceutical excipients and food ingredient compliance; supply chain strategies including sustainable forestry certification; and penetration analysis for pharmaceutical, food, and industrial European customers. |
The global demand for powdered cellulose in EU is estimated to be valued at USD 34.7 million in 2025.
The market size for the demand for powdered cellulose in EU is projected to reach USD 56.5 million by 2035.
The demand for powdered cellulose in EU is expected to grow at a 5.0% CAGR between 2025 and 2035.
The key product types in demand for powdered cellulose in EU are food-grade, pharmaceutical-grade and industrial-grade.
In terms of application (end-use), food & beverage segment to command 37.0% share in the demand for powdered cellulose in EU in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Western Europe Powdered Cellulose Market Analysis by Source, End-use, Functionality, and Country Through 2035
Powdered Cellulose Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Japan Powdered Cellulose Market, By Type, By Application, By Region, and Forecast, 2025 to 2035
Korea Powdered Cellulose Market Analysis by Source, End-use, Functionality, and Region Through 2035
Europe Radiotherapy Patient Positioning Market Size and Share Forecast Outlook 2025 to 2035
Cellulose Diacetate Film Market Size and Share Forecast Outlook 2025 to 2035
Europe Polyvinyl Alcohol Industry Analysis Size and Share Forecast Outlook 2025 to 2035
Cellulose Fiber Market Forecast and Outlook 2025 to 2035
Europe Cruise Market Forecast and Outlook 2025 to 2035
Europium Market Forecast and Outlook 2025 to 2035
Cellulose Derivative Market Size and Share Forecast Outlook 2025 to 2035
Cellulose Film Packaging Market Size and Share Forecast Outlook 2025 to 2035
Eucommia Leaf Extract Market Size and Share Forecast Outlook 2025 to 2035
Europe Massage Therapy Service Market Size and Share Forecast Outlook 2025 to 2035
Cellulose Ether and Derivatives Market Size and Share Forecast Outlook 2025 to 2035
Europe Cement Market Analysis Size and Share Forecast Outlook 2025 to 2035
European Union Tourism Industry Size and Share Forecast Outlook 2025 to 2035
Europe Injection Molding Machines Market Size and Share Forecast Outlook 2025 to 2035
Europe Injection Moulders Market Size and Share Forecast Outlook 2025 to 2035
Europe and MENA Generic Oncology Drug Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA